DCIS consists of a molecularly heterogeneous group of premalignant lesions, with variable risk of invasive progression. Understanding biomarkers for invasive progression could help individualise treatment recommendations based upon tumour biology and reduce overtreatment. As part of the National Cancer Institute’s Human Tumor Atlas Network (HTAN), Dr Siri Strand (Stanford University, CA, USA) and colleagues conducted comprehensive genomic analyses on 2 large DCIS case-control cohorts with a 7.1-year median follow-up (TBCRC 038 and RAHBT) [1,2].
Gene expression analysis of 677 DCIS samples from 481 patients resulted in an 812-gene profile, which was predictive for recurrence (AUC 0.72). Based on the RNA expression of all coding genes, 3 clusters were identified, referred to as ER low, quiescent, and ER high. The ER-low cluster had significantly higher levels of ERBB2 and lower levels of ESR1 compared to quiescent and ER-high clusters. Quiescent cluster lesions were less proliferative and less metabolically active than ER high and ER-low subtypes. Based on DNA copy number aberrations, 6 subtypes of DCIS were identified. Focusing on the stromal component of DCIS from laser capture microdissection, 4 distinct DCIS-associated stromal clusters were identified. A “normal-like” stromal cluster with ECM organisation and PI3K-AKT signalling, a “collagen-rich” stromal cluster, a “desmoplastic” stromal cluster with high fibroblast and total myeloid abundance, mostly associated with macrophages and myeloid dendritic cells (mDC), and an “immune-dense” stromal cluster.
“Comprehensive genomic profiling in 2 independent DCIS cohorts with longitudinal outcomes shows distinct DCIS stromal expression patterns and immune cell composition. RNA expression profiles reveal underlying tumour biology that is associated with later ipsilateral breast events in both cohorts. These studies provide new insight into DCIS biology and will guide the design of diagnostic strategies to prevent invasive progression,” said Dr Strand.
- Strand SH, et al. The Breast PreCancer Atlas DCIS genomic signatures define biology and correlate with clinical outcomes: An analysis of TBCRC 038 and RAHBT cohorts. GS4-07, SABCS 2021 Virtual Meeting, 7–10 December.
- Strand SH, et al. 2021 bioRxiv doi: 1101/2021.06.16.448585.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« BRCA2 linked to inferior outcomes with CDK4/6 inhibitors plus endocrine therapy Next Article
MAPK-pathway alterations are associated with resistance to anti-HER2 therapy »
« BRCA2 linked to inferior outcomes with CDK4/6 inhibitors plus endocrine therapy Next Article
MAPK-pathway alterations are associated with resistance to anti-HER2 therapy »
Table of Contents: SABCS 2021
Featured articles
Early-Stage Breast Cancer
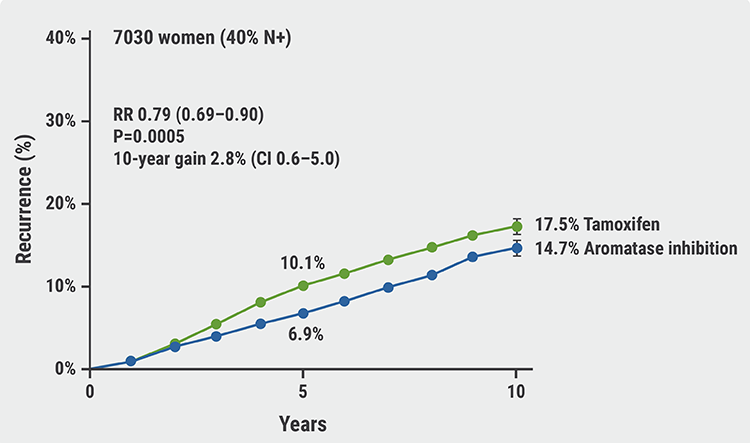
Aromatase inhibitors outperform tamoxifen in premenopausal women
Concurrent taxane plus anthracycline most beneficial in reducing risk of breast cancer
Reduced risk of recurrence with ovarian suppression plus tamoxifen/exemestane
Metformin does not improve outcomes in patients with early-stage breast cancer
Omitting sentinel lymph node biopsy improves arm symptoms
HR-positive/HER2-negative Breast Cancer
Addition of palbociclib to standard endocrine therapy does not improve outcome in adjuvant treatment
The SERD elacestrant improves outcomes for patients unresponsive to endocrine therapy
Consistent overall survival benefit of ribociclib in advanced breast cancer
Premenopausal women benefit from adjuvant chemotherapy next to endocrine therapy
Promising anti-tumour activity of the CDK7-inhibitor samuraciclib plus fulvestrant
ctDNA is prognostic and predictive for response to ribociclib plus letrozole
Early switch to fulvestrant plus palbociclib beneficial for patients with ESR1 mutation
Triple-Negative Breast Cancer
Single-cell spatial analysis can predict response to neoadjuvant immunotherapy
Neoadjuvant pembrolizumab plus chemotherapy benefits event-free survival in TNBC
Early use of ctDNA testing can identify likelihood of relapse in TNBC
Pembrolizumab plus chemotherapy benefits patients with combined positive score ≥10
Neratinib plus trastuzumab plus fulvestrant shows encouraging clinical activity
Phase 1–3 Trials
Datopotamab deruxtecan shows promising anti-tumour activity
Trastuzumab deruxtecan outperforms trastuzumab emtansine
Nivolumab plus ipilimumab serve promising dual checkpoint inhibition
Entinostat plus exemestane improves progression-free survival in Chinese patients
Efficacy of pyrotinib plus capecitabine confirmed in previously treated patients
Basic and Translational Research
Using genomics to match treatments improves outcomes
Loss of ASXL1 tumour suppressor promotes resistance to CDK4/6 inhibitors
Inducers of ferroptosis are potential drugs to target p53-mutated TNBC cells
MAPK-pathway alterations are associated with resistance to anti-HER2 therapy
Genomic signatures of DCIS define biology and correlate with clinical outcomes
BRCA2 linked to inferior outcomes with CDK4/6 inhibitors plus endocrine therapy
Miscellaneous
Olaparib is well tolerated as an additional treatment
Race effects the likelihood to develop lymphoedema following breast cancer treatment
Sentinel lymph node staging is non-inferior to complete axillary lymph node dissection
One in 7 breast cancers detected during screening are overdiagnosed
Related Articles
January 31, 2022
Aromatase inhibitors outperform tamoxifen in premenopausal women
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

