Immune checkpoint inhibitors are effective in early and advanced TNBC. However, only a minority of patients benefit from it, making precision immuno-oncology a major unmet need. Imaging mass cytometry enables high dimensional tissue imaging at subcellular resolution for assessment of TNBC ecosystems, providing information on cell type composition, functional status, and spatial organisation. Dr Giampaolo Bianchini (Ospedale San Raffaele, Italy) and colleagues investigated whether imaging mass cytometry could assist in the identification of ideal candidates for this therapeutic approach. They performed imaging mass cytometry analysis in the context of the phase 3 NeoTRIPaPDL1 trial (NCT02620280), which was designed to evaluate the addition of atezolizumab to the chemotherapeutics carboplatin and nab-paclitaxel as neoadjuvant therapy in patients with early high-risk and locally advanced TNBC.
Forty-three protein-spanning cancer cells and the tumour microenvironment were assessed on pre-treatment, formalin-fixed, paraffin-embedded biopsies of 243 of the 280 enrolled patients. For each sample, 3 high-dimensional images were generated that encompassed the tumour, tumour-stroma interface, and adjacent stroma. The association of protein expression was assessed for epithelial and tumour-microenvironment cells, cell phenotypes, and the spatial tissue organisation with pathological complete response rate. Dr Bianchini presented the results [1].
By supervised clustering, 37 cell phenotypes were defined. PD-L1-positive tumours, high stromal tumour-infiltrating lymphocytes, and TNBC type were characterised by extreme heterogeneity and unique cell-type and spatial tumour microenvironment composition. Bulk protein expression analysis delivered only limited predictive information because it does not consider the cell compartment in which each protein is expressed. Several biomarkers demonstrated a significant association with pathological complete response rate. For example, high density of PD-L1-positive and IDO-positive antigen presenting cells, as well as high density of CD56-positive epithelial cells, was associated with higher pathological complete response rate in patients who received atezolizumab plus chemotherapy, but not in patients who only received chemotherapy (OR 4.5; p<0.001). In addition, high degree of spatial connectivity between epithelial cells and specific tumour-microenvironment cells correlated with a significant increase in the pathological complete response rate after atezolizumab.
“Our results demonstrate that imaging mass cytometry is feasible in a large, randomised trial and provides a comprehensive overview of TNBC at a single-cell level with special resolution, paving the way for its broad implementation in cancer research to aid precision immunology,” concluded Bianchini. “Information on spatial data and on the interactions among specific cells in the tumour microenvironment might be informative about the benefit provided by an immune checkpoint inhibitor such as atezolizumab in addition to chemotherapy. However, all these findings will require independent validation.”
- Bianchini G, et al. Single-cell spatial analysis by imaging mass cytometry and immunotherapy response in triple-negative breast cancer (TNBC) in the NeoTRIPaPDL1 trial. GS1-00, SABCS 2021 Virtual Meeting, 7–10 December.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Neoadjuvant pembrolizumab plus chemotherapy benefits event-free survival in TNBC Next Article
Efficacy of pyrotinib plus capecitabine confirmed in previously treated patients »
« Neoadjuvant pembrolizumab plus chemotherapy benefits event-free survival in TNBC Next Article
Efficacy of pyrotinib plus capecitabine confirmed in previously treated patients »
Table of Contents: SABCS 2021
Featured articles
Early-Stage Breast Cancer
Aromatase inhibitors outperform tamoxifen in premenopausal women
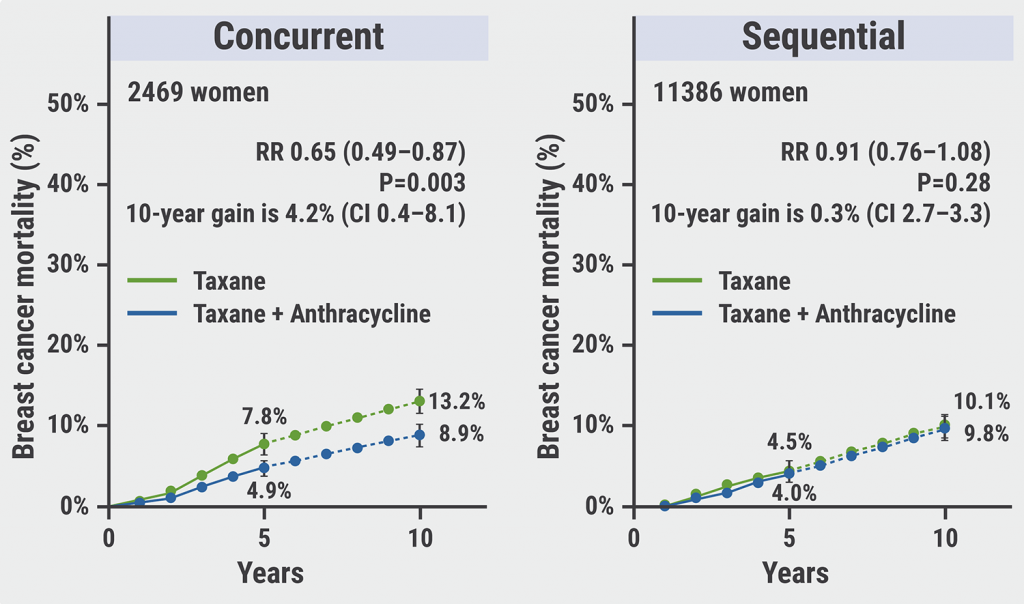
Concurrent taxane plus anthracycline most beneficial in reducing risk of breast cancer
Reduced risk of recurrence with ovarian suppression plus tamoxifen/exemestane
Metformin does not improve outcomes in patients with early-stage breast cancer
Omitting sentinel lymph node biopsy improves arm symptoms
HR-positive/HER2-negative Breast Cancer
Addition of palbociclib to standard endocrine therapy does not improve outcome in adjuvant treatment
The SERD elacestrant improves outcomes for patients unresponsive to endocrine therapy
Consistent overall survival benefit of ribociclib in advanced breast cancer
Premenopausal women benefit from adjuvant chemotherapy next to endocrine therapy
Promising anti-tumour activity of the CDK7-inhibitor samuraciclib plus fulvestrant
ctDNA is prognostic and predictive for response to ribociclib plus letrozole
Early switch to fulvestrant plus palbociclib beneficial for patients with ESR1 mutation
Triple-Negative Breast Cancer
Single-cell spatial analysis can predict response to neoadjuvant immunotherapy
Neoadjuvant pembrolizumab plus chemotherapy benefits event-free survival in TNBC
Early use of ctDNA testing can identify likelihood of relapse in TNBC
Pembrolizumab plus chemotherapy benefits patients with combined positive score ≥10
Neratinib plus trastuzumab plus fulvestrant shows encouraging clinical activity
Phase 1–3 Trials
Datopotamab deruxtecan shows promising anti-tumour activity
Trastuzumab deruxtecan outperforms trastuzumab emtansine
Nivolumab plus ipilimumab serve promising dual checkpoint inhibition
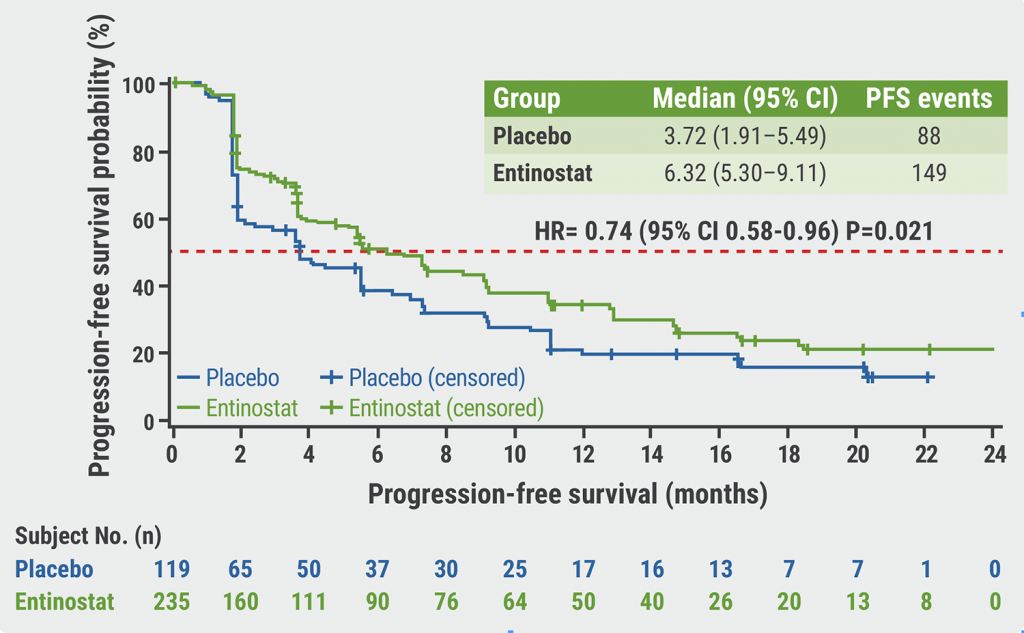
Entinostat plus exemestane improves progression-free survival in Chinese patients
Efficacy of pyrotinib plus capecitabine confirmed in previously treated patients
Basic and Translational Research
Using genomics to match treatments improves outcomes
Loss of ASXL1 tumour suppressor promotes resistance to CDK4/6 inhibitors
Inducers of ferroptosis are potential drugs to target p53-mutated TNBC cells
MAPK-pathway alterations are associated with resistance to anti-HER2 therapy
Genomic signatures of DCIS define biology and correlate with clinical outcomes
BRCA2 linked to inferior outcomes with CDK4/6 inhibitors plus endocrine therapy
Miscellaneous
Olaparib is well tolerated as an additional treatment
Race effects the likelihood to develop lymphoedema following breast cancer treatment
Sentinel lymph node staging is non-inferior to complete axillary lymph node dissection
One in 7 breast cancers detected during screening are overdiagnosed
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy