Clinical and epidemiologic evidence has linked hyperinsulinemia, insulin resistance, obesity, and diabetes to poor breast cancer outcomes. Metformin is an inexpensive, widely available, and well-tolerated drug that promotes modest weight loss and lowers insulin. In addition, metformin is a potential anti-cancer agent; indirectly by lowering the circulating insulin levels and directly by activating adenosine monophosphate-activated protein kinase (AMPK) [1]. CCTGMA.32 (NCT01101438) is a phase 3, randomised, double-blind, placebo-controlled adjuvant trial of metformin versus placebo in early-stage breast cancer. Dr Pamela Goodwin (Lunenfeld-Tanenbaum Research Institute, Canada) presented the results [2].
After standard therapy, 3,649 non-diabetic patients were randomised to metformin (850 mg twice daily, 5 years) or placebo. After the second interim analysis (median follow-up of 29.5 months), intervention was stopped for patients who were HR-negative (n=1,116). In HR-positive patients, the incidence of both invasive disease-free survival (IDSF) events and overall survival (OS) events were evenly distributed between both treatment arms at 96 months of follow-up (IDSF: HR 1.01; 95% CI 0.84–1.21; P=0.93; OS: HR 1.10; 95% CI 0.86–1.41; P=0.47).
“Therefore, metformin should not be used as an adjuvant breast cancer treatment in this population,” concluded Dr Goodwin. “Of note, this recommendation should not be extrapolated to the use of metformin to treat diabetes in breast cancer patients.”
- Goodwin PJ, et al. Clin Oncol. 2009;27:3271–3273.
- Goodwin PJ, et al. CCTGMA.32, a phase III randomized double-blind placebo controlled adjuvant trial of metformin (MET) vs placebo (PLAC) in early breast cancer (BC): Results of the primary efficacy analysis (clinical trials.gov NCT01101438). GS1-08, SABCS 2021 Virtual Meeting, 7–10 December.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Omitting sentinel lymph node biopsy improves arm symptoms Next Article
Reduced risk of recurrence with ovarian suppression plus tamoxifen/exemestane »
« Omitting sentinel lymph node biopsy improves arm symptoms Next Article
Reduced risk of recurrence with ovarian suppression plus tamoxifen/exemestane »
Table of Contents: SABCS 2021
Featured articles
Early-Stage Breast Cancer
Aromatase inhibitors outperform tamoxifen in premenopausal women
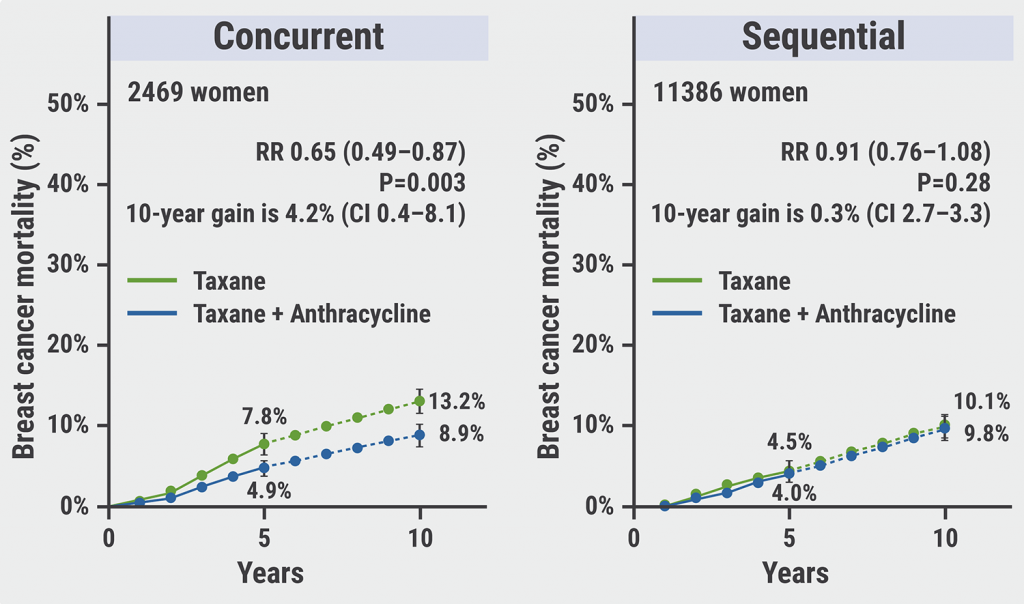
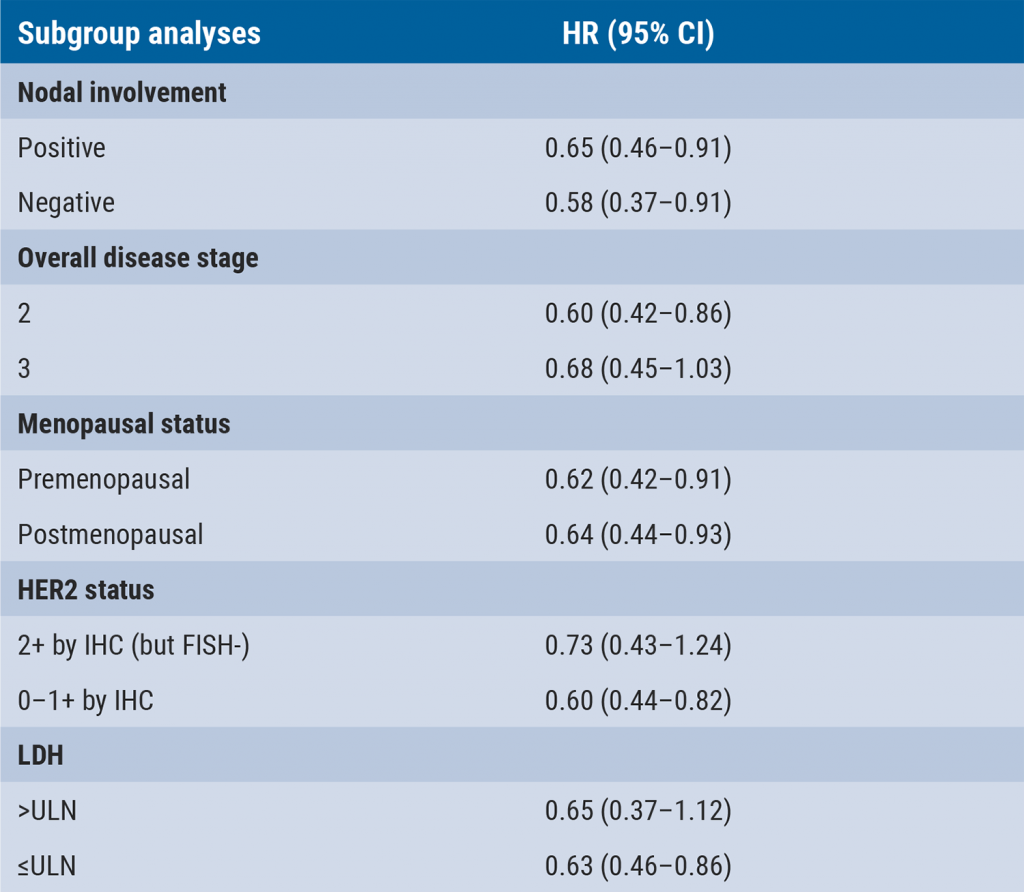
Concurrent taxane plus anthracycline most beneficial in reducing risk of breast cancer
Reduced risk of recurrence with ovarian suppression plus tamoxifen/exemestane
Metformin does not improve outcomes in patients with early-stage breast cancer
Omitting sentinel lymph node biopsy improves arm symptoms
HR-positive/HER2-negative Breast Cancer
Addition of palbociclib to standard endocrine therapy does not improve outcome in adjuvant treatment
The SERD elacestrant improves outcomes for patients unresponsive to endocrine therapy
Consistent overall survival benefit of ribociclib in advanced breast cancer
Premenopausal women benefit from adjuvant chemotherapy next to endocrine therapy
Promising anti-tumour activity of the CDK7-inhibitor samuraciclib plus fulvestrant
ctDNA is prognostic and predictive for response to ribociclib plus letrozole
Early switch to fulvestrant plus palbociclib beneficial for patients with ESR1 mutation
Triple-Negative Breast Cancer
Single-cell spatial analysis can predict response to neoadjuvant immunotherapy
Neoadjuvant pembrolizumab plus chemotherapy benefits event-free survival in TNBC
Early use of ctDNA testing can identify likelihood of relapse in TNBC
Pembrolizumab plus chemotherapy benefits patients with combined positive score ≥10
Neratinib plus trastuzumab plus fulvestrant shows encouraging clinical activity
Phase 1–3 Trials
Datopotamab deruxtecan shows promising anti-tumour activity
Trastuzumab deruxtecan outperforms trastuzumab emtansine
Nivolumab plus ipilimumab serve promising dual checkpoint inhibition
Entinostat plus exemestane improves progression-free survival in Chinese patients
Efficacy of pyrotinib plus capecitabine confirmed in previously treated patients
Basic and Translational Research
Using genomics to match treatments improves outcomes
Loss of ASXL1 tumour suppressor promotes resistance to CDK4/6 inhibitors
Inducers of ferroptosis are potential drugs to target p53-mutated TNBC cells
MAPK-pathway alterations are associated with resistance to anti-HER2 therapy
Genomic signatures of DCIS define biology and correlate with clinical outcomes
BRCA2 linked to inferior outcomes with CDK4/6 inhibitors plus endocrine therapy
Miscellaneous
Olaparib is well tolerated as an additional treatment
Race effects the likelihood to develop lymphoedema following breast cancer treatment
Sentinel lymph node staging is non-inferior to complete axillary lymph node dissection
One in 7 breast cancers detected during screening are overdiagnosed
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com