Glucocorticoids are highly effective in treating RA. However, the potential systemic side effects limit the long-term use of these drugs. ABBV-3373 is an antibody-drug conjugate consisting of adalimumab linked to a proprietary GRM, which is designed to use the favourable aspects of a TNF inhibitor and a glucocorticoid. GRMs can selectively induce a receptor conformation in a way that only activates a subset of downstream signalling pathways, thereby combining agonistic and antagonistic properties of glucocorticoids, depending on the tissue in which it is expressed. This targeted approach may potentially dampen systemic side effects [2].
In the current trial, RA patients were randomised 2:1 to placebo (n=96) or one of the experimental conditions: in-trial adalimumab (n=17; 80 mg subcutaneous every other week) or ABBV-3373 (n=31; 100 mg intravenous every other week). Efficacy and safety data of ABBV-3373 were compared with data of 242 historical adalimumab recipients, in-trial adalimumab users, and placebo users. Primary endpoint was the change from baseline 28-joint Disease Activity Score based on C-reactive protein (DAS28–CRP) scores at week 12.
The results were presented by Dr Frank Buttgereit (Charité University Medicine, Germany) and demonstrated patient benefits of ABBV-3373 over historical adalimumab users and placebo, represented as mean change on DAS28–CRP scores after 12 weeks of treatment: -2.65 for ABBV-3373 versus -2.13 for historical adalimumab (P<0.05) and versus -0.91 for placebo (P<0.001). No significant difference between DAS28–CRP mean changes of ABBV-3373 and in-trial adalimumab users was observed (see Figure).
Figure: Change from baseline in DAS28–CRP at week 12 [1]
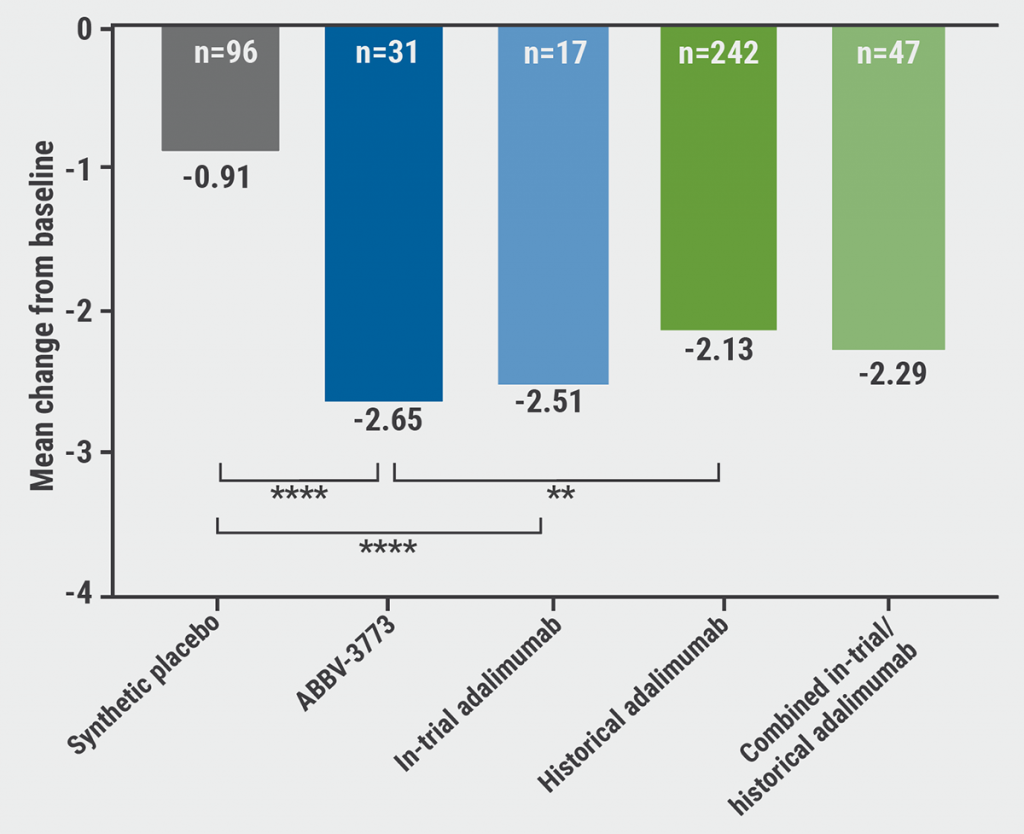
DAS28–CRP, 28-joint disease activity score based on C-reactive protein.
** P<0.05; **** P<0.001
Similar positive results were obtained for secondary outcome measures: DAS28 based on Erythrocyte Sedimentation Rate, Crohn’s Disease Activity Index (CDAI), Simplified Disease Activity Index for RA (SDAI), ACR responses 20/50/70, and Health Assessment Questionnaire–Disability Index (HAQ–DI). Fewer AEs were reported in patients treated with ABBV-3373 (11, 35.5%) compared with in-trial adalimumab recipients (12, 70.6%). Nonetheless, 4 AEs in the ABBV-3373 population were considered serious: non-cardiac chest pain, pneumonia, upper respiratory tract infection, and anaphylactic shock (all n=1). Serious AEs were not reported in the in-trial adalimumab population. The safety profile of ABBV-3373 was fairly similar to that of adalimumab.
Dr Buttgereit argued that these findings demonstrate the potential of ABBV-3373 to provide benefits for RA patients compared with adalimumab. “All RA patients could benefit from the targeted delivery of this TNF inhibitor linked to a GRM”.
- Buttgereit F, et al. Efficacy and Safety of ABBV-3373, a Novel Anti-TNF Glucocorticoid Receptor Modulator Antibody Drug Conjugate, in Patients with Moderate to Severe Rheumatoid Arthritis Despite Methotrexate Therapy: A Phase 2a Proof of Concept Study. OP0115, EULAR 2021 Virtual Congress, 2–5 June.
- Meijer OC, et al. Ann Endocrinol (Paris). 2018;79(3):107-11.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Emerging therapies and future treatment directions in osteoarthritis Next Article
The risks of polypharmacy in RA »
« Emerging therapies and future treatment directions in osteoarthritis Next Article
The risks of polypharmacy in RA »
Table of Contents: EULAR 2021
Featured articles
COVID-19 Update
Rituximab or JAK inhibitors increase the risk of severe COVID-19
Updates on COVID-19 vaccines in patients with rheumatic disease
Immunomodulatory therapies for severe COVID-19: literature update
New Developments in Rheumatoid Arthritis
JAK inhibitors and bDMARDs not associated with increased risk of serious infections in RA
Remote management of RA is a feasible alternative for outpatient follow-up
TOVERA: Ultrasound is a promising biomarker of early treatment response
The risks of polypharmacy in RA
ABBV-3373: A potential new therapeutic agent for RA
JAK inhibitors and bDMARDs show comparable effectiveness
Spondyloarthritis: Progression in Therapies
SELECT-AXIS: 64-week results of upadacitinib in active ankylosing spondylitis
Guselkumab efficacious in PsA patients with inadequate response to TNF inhibition
Faecal microbiota transplantation not effective in active peripheral PsA
Risankizumab meets primary and ranked secondary endpoints in PsA
Prognostic factors for minimal disease activity in early psoriatic arthritis revealed
Imaging in Large-Vessel Vasculitis
PET/CT is a reliable measure of disease activity in LVV, but does not predict future relapses
Ultrasound is useful for disease monitoring in giant cell arteritis
Prevention in Rheumatic Diseases
Air pollution predicts decreased response to biological treatment in rheumatic diseases
Passive smoking associated with an increased risk of RA
Gene-Environment Interaction in Gout
Gene-diet and gene-weight interactions associated with the risk of gout
What Is New in Systemic Lupus Erythematosus
Intensified treatment regimen of anifrolumab for lupus nephritis is promising
Systemic lupus erythematosus: increased risk of severe infection
Juvenile Idiopathic Arthritis and Osteoarthritis
Efficacy and safety of secukinumab in juvenile idiopathic arthritis
Emerging therapies and future treatment directions in osteoarthritis
Related Articles
July 21, 2022
New treatments in osteoarthritis
February 4, 2020
Hand OA: low-dose corticosteroids improve symptoms
November 21, 2024
XG005 relieves OA symptoms in phase 2b study
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

