Efficacy and safety of secukinumab, a human monoclonal antibody targeting IL-17, has been demonstrated in adult patients with PsA and radiographic and non-radiographic axial spondyloarthritis [2-4]. JUNIPERA (NCT03031782) aimed to evaluate the efficacy and safety of secukinumab in ERA and JPsA patients (aged 2–18) with a history of inadequate response or intolerance to ≥1 NSAID or ≥1 DMARD. If patients (n=86) reached juvenile idiopathic arthritis (JIA)-ACR30 at the end of a 12-week open-label secukinumab 75/150 mg subcutaneous first treatment period (every week first 4 weeks, then every 4 weeks), they were randomised to secukinumab every 4 weeks (n=37) or placebo (n=38). After the occurrence of a flare in the second treatment period, the patient was moved to another open-label secukinumab treatment period. The primary endpoint was time to disease flare of patients on secukinumab versus placebo in the second treatment period.
Time to flare was significantly longer in the secukinumab arm compared with the placebo arm (HR 0.28; 95% CI 0.13-0.63; P<0.001) (see Figure). The number of flares was lower in the treatment arm (secukinumab 10 vs placebo 21) and JIA-ACR30 was maintained more often for secukinumab-treated patients (89.2%) than for placebo patients (64.9%) after the second treatment period. Dr Nicola Ruperto (IRCCS Istituto G. Gaslini, Italy) argued that the median time to flare of 453 days in the placebo arm indicated a prolonged biological effect of the first treatment period. Complete resolution of enthesitis occurred in 73.9% of the ERA patients after the first treatment period. Adverse events (AEs) were reported in 91.7% of the secukinumab patients and 92.1% of the placebo patients, including 7 and 4 non-fatal serious AEs, respectively. These findings also support the close pathophysiological link between ERA and adult spondyloarthropathy pattern arthritis where IL-17 blockers are also effective.
Figure: Primary endpoint of time to disease flare for secukinumab versus placebo [1]
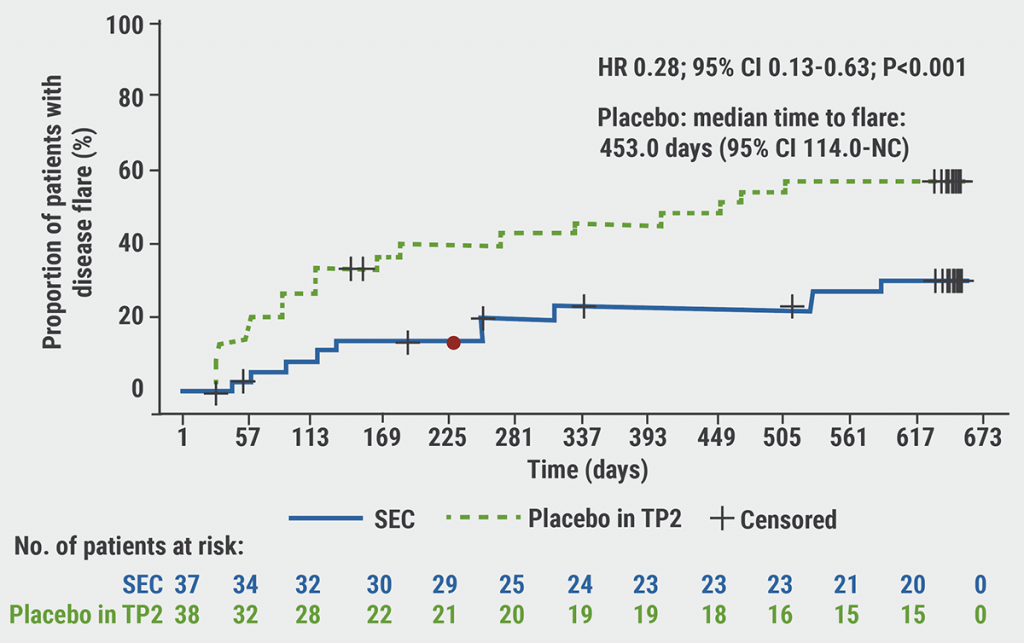
SEC, secukinumab; TP, treatment period.
- Ruperto N, et al. Efficacy and Safety of Secukinumab in Enthesitis-related Arthritis and Juvenile Psoriatic Arthritis: Primary Results from a Randomised, Double-blind; Placebo-controlled, Treatment Withdrawal; Phase 3 Study (JUNIPERA). LB0004, EULAR 2021 Virtual Congress, 2–5 June.
- McInnes IB, et al. Lancet. 2015;386:1137–46.
- Baeten D, et al. N Engl J Med. 2015;373:2534–48.
- Deodhar A, et al. Arthritis Rheumatol. 2021;73:110–20.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Intensified treatment regimen of anifrolumab for lupus nephritis is promising Next Article
Rituximab or JAK inhibitors increase the risk of severe COVID-19 »
« Intensified treatment regimen of anifrolumab for lupus nephritis is promising Next Article
Rituximab or JAK inhibitors increase the risk of severe COVID-19 »
Table of Contents: EULAR 2021
Featured articles
COVID-19 Update
Rituximab or JAK inhibitors increase the risk of severe COVID-19
Updates on COVID-19 vaccines in patients with rheumatic disease
Immunomodulatory therapies for severe COVID-19: literature update
New Developments in Rheumatoid Arthritis
JAK inhibitors and bDMARDs not associated with increased risk of serious infections in RA
Remote management of RA is a feasible alternative for outpatient follow-up
TOVERA: Ultrasound is a promising biomarker of early treatment response
The risks of polypharmacy in RA
ABBV-3373: A potential new therapeutic agent for RA
JAK inhibitors and bDMARDs show comparable effectiveness
Spondyloarthritis: Progression in Therapies
SELECT-AXIS: 64-week results of upadacitinib in active ankylosing spondylitis
Guselkumab efficacious in PsA patients with inadequate response to TNF inhibition
Faecal microbiota transplantation not effective in active peripheral PsA
Risankizumab meets primary and ranked secondary endpoints in PsA
Prognostic factors for minimal disease activity in early psoriatic arthritis revealed
Imaging in Large-Vessel Vasculitis
PET/CT is a reliable measure of disease activity in LVV, but does not predict future relapses
Ultrasound is useful for disease monitoring in giant cell arteritis
Prevention in Rheumatic Diseases
Air pollution predicts decreased response to biological treatment in rheumatic diseases
Passive smoking associated with an increased risk of RA
Gene-Environment Interaction in Gout
Gene-diet and gene-weight interactions associated with the risk of gout
What Is New in Systemic Lupus Erythematosus
Intensified treatment regimen of anifrolumab for lupus nephritis is promising
Systemic lupus erythematosus: increased risk of severe infection
Juvenile Idiopathic Arthritis and Osteoarthritis
Efficacy and safety of secukinumab in juvenile idiopathic arthritis
Emerging therapies and future treatment directions in osteoarthritis
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

