https://doi.org/10.55788/f299ddd4
As Dr Hafsah Nabi (Rigshospitalet, Denmark) pointed out, biosimilars play an important role in rheumatology, especially due to their economic advantage [1]. Evidence for their use is mainly based on randomised controlled trials. “We are missing data across indications in older age and in patients with complex comorbidities,” Dr Nabi said. In addition, data regarding the outcomes after switching from one infliximab biosimilar to a second one is limited.
Denmark is known for its biosimilar success. Since 2015, switches have been mandatory, at the beginning from the original product to a biosimilar, but later from 1 biosimilar to another. In her study, Dr Nabi assessed the effectiveness of switching infliximab biosimilars CT-P13 to GP1111 among patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA). Patients were analysed in 2 cohorts: those who had previously switched from the originator (originator-experienced) and those who were originator-naïve. Data from the DANBIO registry linked with national patient registries were used, the latter to identify comorbidities.
The main outcome measures in the 2 groups were 1-year GP1111 treatment retention and changes in disease activity 4 months before versus 4 months after the switch. The researchers also identified clinical factors at baseline associated with GP1111 treatment withdrawal.
Included were 1,605 patients (685 RA, 314 PsA, and 606 axSpA) with a median disease duration of 9 years, of which 1,171 were originator-naïve. The originator-naïve group tended to be younger, with lower disease duration, but higher disease activity.
One-year retention rates of GP1111 were 92% (95% CI 90-95%) in the originator-experienced and 83% (95% CI 81-85%) in the originator-naïve, respectively. Changes in disease activity 4 months pre- and post-switch were close to zero for all disease activity measures (e.g. Disease Activity Score in 28 joints [DAS28], Ankylosing Spondylitis Disease Activity Score [ASDAS], pain Visual Analogue Scale [VAS]).
Factors associated with higher GP1111 retention rates were being originator-experienced compared to originator-naïve in the fully adjusted cohort (HR=0.36; 95% CI 0.2-0.7) and lower disease activity at baseline.
“Overall, the 1-year retention rates after biosimilar-to biosimilar infliximab switch was high. Moreover, the disease activity remained stable 4 months before and after switch,” Dr Nabi summarised. The fact that retention was higher in originator-experienced patients and in patients with low disease activity suggests that outcomes are predominantly affected by patient- rather than drug-related factors.
- Nabi H. Biosimilar-to-Biosimilar Switching in Routine Care – Results on >1,600 Patients with Inflammatory Arthritis in the DANBIO Registry. 1112, ACR Convergence 2022, 10–14 November, Philadelphia, USA.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« New analysis assesses the safety of tofacitinib regarding extended MACE Next Article
Denosumab in erosive hand arthritis: Structure repair of interphalangeal joints seems possible »
« New analysis assesses the safety of tofacitinib regarding extended MACE Next Article
Denosumab in erosive hand arthritis: Structure repair of interphalangeal joints seems possible »
Table of Contents: ACR 2022
Featured articles
Bruton’s tyrosine kinase inhibition: a novel treatment option for Sjögren’s syndrome?
Early treatment: a key to improved outcomes in polyarticular JIA
ACR 2022 Congress Round-Up
Glucocorticoids in rheumatic diseases: Is there a skeletal sparing dose?
Bimekizumab: Robust 1-year results in treating psoriatic arthritis
Stimulation of PD-1: a new concept to treat RA
Denosumab in erosive hand arthritis: Structure repair of interphalangeal joints seems possible
High retention rates after switching between infliximab biosimilars
New analysis assesses the safety of tofacitinib regarding extended MACE
Lack of vaccination results in a higher frequency of pre-term births in pregnant women with rheumatic disease and COVID-19
Ankylosing spondylitis: Combining biologics with NSAID does not imply reduced radiographic progression
Diet and exercise programme: a successful OA strategy in a community-based setting
Bruton’s tyrosine kinase inhibition: a novel treatment option for Sjögren’s syndrome?
A novel risk score helps identify interstitial lung disease in patients with systemic sclerosis
Early treatment: a key to improved outcomes in polyarticular JIA
TYK2 inhibition shows potential as a treatment for SLE
Belimumab efficacious in cutaneous lupus erythematosus
No increased cancer risk in patients with rheumatic diseases and prior malignancy treated with novel therapies
Related Articles
February 4, 2020
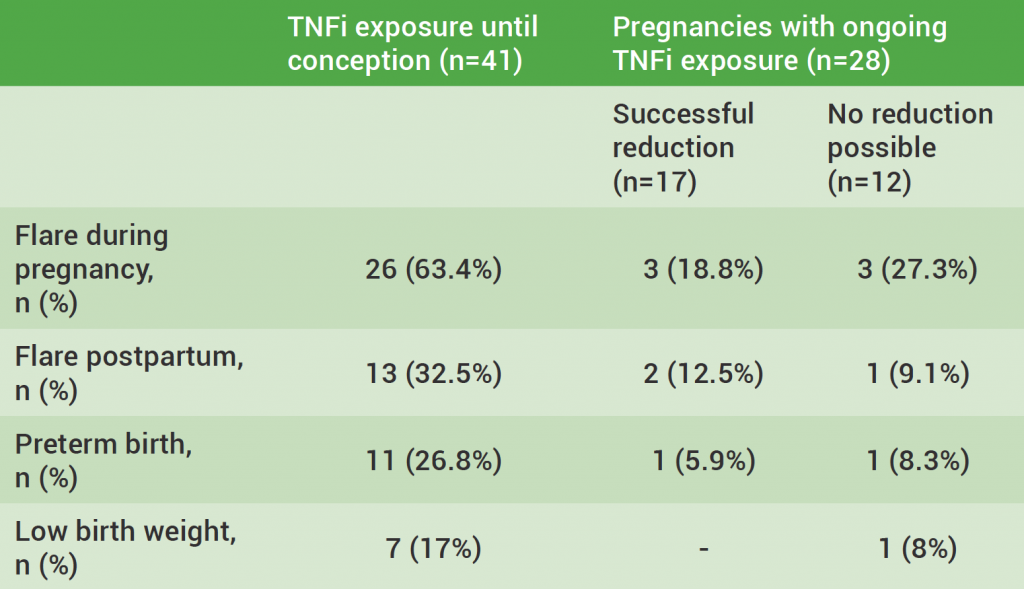
TNFi for RA during pregnancy – to stop or not to stop?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

