https://doi.org/10.55788/3eaa24c1
Prof. Njira Lugogo (University of Michigan, MI, USA) presented a prespecified, on-treatment analysis of responses to tezepelumab using data from the completed phase 3, double-blind, placebo-controlled NAVIGATOR trial (NCT03347279) [1]. Participants aged 12–80 years were randomised to receive either subcutaneous injections of 210 mg tezepelumab (n=528) or subcutaneous placebo (n=531) every 4 weeks for 52 weeks while continuing to take their medium- or high-dose corticosteroid inhalers and at least 1 other asthma-control medication during the study.
The primary endpoint of annual asthma exacerbation rate at week 52 was met (see Figure). Across response criteria, the proportion of responders was higher in the tezepelumab than in the placebo group for exacerbation reduction (85.4% vs 67.5%; OR 2.73; 95% CI 2.04–3.90); Asthma Control Questionnaire (ACQ)-6 total score (86.9% vs 76.6%; OR 2.05; 95% CI 1.98–3.77); an improvement from baseline pre-bronchodilator forced expiratory volume in 1 second (FEV1; 60.3% vs 49.9%; OR 1.52; 95% CI 1.15–2.01); and in Clinical Global Impression of Change (CGI-C) score (81.5% vs 67.7%; OR 2.25; 95% CI 1.61–3.14). The proportion of complete responders (those who achieved significant improvement on all measures) was also greater in the tezepelumab group (46.2% vs 24.3%; OR 2.83; 95% CI 2.10–3.82).
Figure: Proportions of participants meeting clinical response criteria were higher with tezepelumab than placebo at week 52 in the on-treatment population [1]
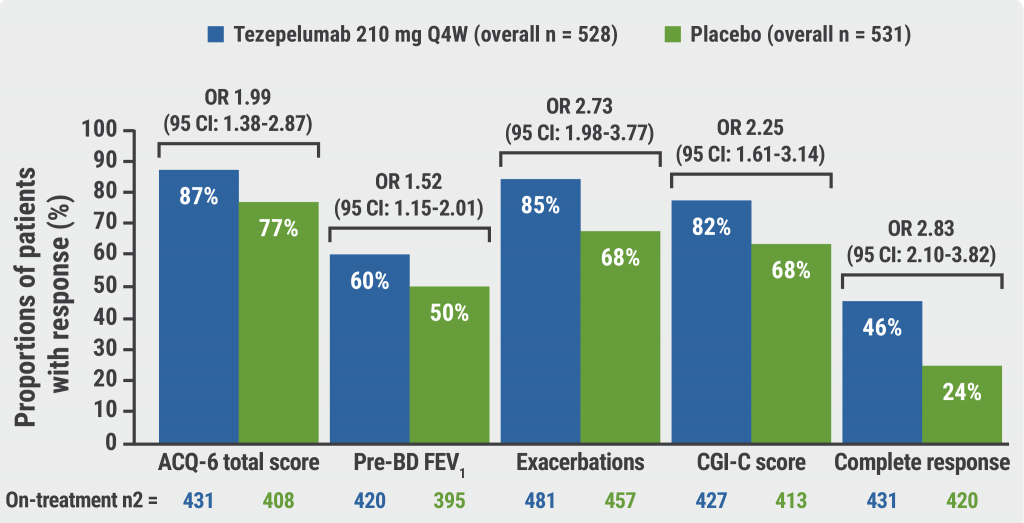
ACQ, Asthma Control Questionnaire; CI, confidence interval; CGI-C, Clinical Global Impression of Change; pre-BD FEV1, pre-bronchodilator forced expiratory volume in 1 second; OR, odds ratio; Q4W, every 4 weeks.
Prof. Lugogo concluded that “overall, these results add an important patient-level perspective to the primary study results.” Across each measure, tezepelumab recipients were more likely to have a response; the greatest difference observed was for exacerbation reduction. In addition, 48% of participants receiving tezepelumab had a complete response and achieved significant and clinically relevant improvements in all 4 response measures.
- Lugogo N, et al. Clinical Responses to Treatment with Tezepelumab Among Patients with Severe, Uncontrolled Asthma in the Phase 3 NAVIGATOR Study. Session B93, ATS International Conference 2022, San Francisco, CA, USA, 13–18 May.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« High-intensity interval training slashes daily corticosteroids in asthma Next Article
Type 2 asthma in children managed by dupilumab, despite atopic comorbidities »
« High-intensity interval training slashes daily corticosteroids in asthma Next Article
Type 2 asthma in children managed by dupilumab, despite atopic comorbidities »
Table of Contents: ATS 2022
Featured articles
Letter from the Editor
COVID-19
Nebulised aviptadil “futile” in I-SPY COVID-19 trial
Lung transplantation after COVID-19-associated ARDS
Mesenchymal stem cells offer no benefit in COVID-19
Alpha-1 antitrypsin for ARDS secondary to severe COVID-19
Frailty prevalent 5 months following hospitalisation for COVID-19
Paediatric long COVID lacks definitions
Asthma Clinical Trial Updates
MANDALA and DENALI pattern success for albuterol-budesonide in asthma
ACOUSTICS data sounds good for adolescent asthma exacerbations
Type 2 asthma in children managed by dupilumab, despite atopic comorbidities
NAVIGATOR steers asthma patients to tezepelumab
High-intensity interval training slashes daily corticosteroids in asthma
Chronic Obstructive Pulmonary Disease
Three’s a crowd for triple therapy in COPD
Higher 1-year COPD mortality after hospitalisation for White patients
Reducing dyspnoea in chronic lung disease through weight loss
CT-evident mucus plugs in COPD associated with death
Home-based rehabilitation improves COPD: a randomised study
Highlighted Advances
Novel P2X3 antagonist can SOOTHE chronic cough
Colistimethate sodium PROMISing for non-cystic fibrosis bronchiectasis
Is avacopan better than prednisone for respiratory ANCA-associated vasculitis outcomes?
PAGANINI phase 2b data promising for eliapixant
POISE-3: Tranexamic acid for non-cardiac surgery
Obstructive sleep apnoea in most children with pulmonary hypertension
No screening evidence for COPD
Novel PDE4B inhibitor offers breakthrough for IPF
Hydrocortisone does not help preterm infants
CPAP temporarily supports pulmonary oxygenation in morbidly obese patients
ISAACC trial: CPAP controls blood pressure in ACS patients with severe OSA
Related Articles
September 17, 2021
Circadian system implicated in asthma worsening at night
October 30, 2022
Fish oil or vitamin D during pregnancy can prevent croup
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

