Featured video: INFORM study examining use of precision medicine for pediatric cancers with poor prognosis.
In-depth tumour molecular characterisation of children and adolescents who have relapsed after initial therapy, and for whom there are no established treatment concepts available, can offer diagnostic insight and potential novel therapeutic approaches.
Prof. Cornelis van Tilburg (Hopp Children’s Cancer Center, Germany) presented the INdividualized therapy FOr children with Relapsed Malignancies (INFORM) registry study [1]. INFORM evaluates the clinical potential of precision oncology in children by identifying molecular targets of (off-label) treatment, identifying potential biomarkers for clinical trials or other uses, and providing additional diagnostic precision (diagnostic refinement). INFORM gives children a chance to benefit from off-label (adult oncology) targeted therapies and enrolment in biomarker-driven clinical trials. This presentation is the first time a clinical outcome in a real-world setting of a large multi-national personalised paediatric oncology platform was assessed.
INFORM included 72 sites in 8 countries. All tumour material was molecularly analysed in Heidelberg and discussed by a central board together with the treating paediatric oncologist. An algorithm developed by the project assigned patients to a 7-step, pre-defined priority scale, based on draggability, alteration type, and specific evidence/relevance. Treatment was decided by the treating physician, considering the available clinical trials, off-label options suggested by the algorithm, and other (conventional) therapy. Documentation and clinical follow-up were centrally documented by INFORM.
A total of 526 children were analysed, of which 149 received matching targeted drugs; the algorithm was unable to match 377 patients to any targeted drug. Twenty patients qualified as the highest priority level and were more likely to receive subsequent treatment. Seventeen children (3.2%) were included in clinical trials. Forty children (7.6%) were identified to have potential cancer predisposition syndromes; consequently, their families were offered genetic counselling. Among the children with brain tumours, molecular analyses provided diagnostic refinement in 8%.
In a subgroup analysis of children whose tumours ranked “very high priority level” for a matching targeted drug, an extended and significant progression-free survival was observed (204 days vs 114 days; P=0.0093, see Figure), indicating the algorithm accurately predicts responders to a given therapy.
Figure. Progression-free survival (PFS) of children whose tumours ranked “very high priority level” for a matching targeted drug [1]
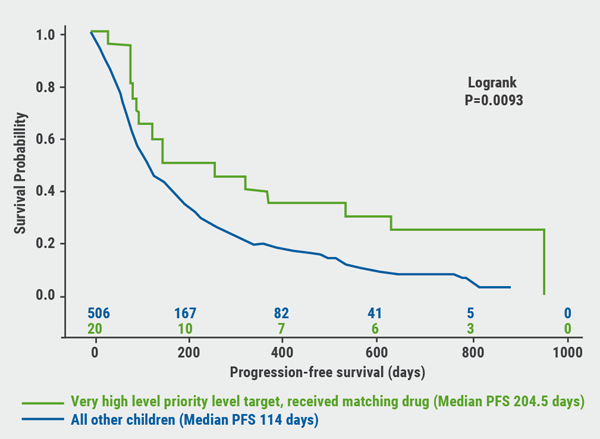
Although this study is limited in size and consists of a highly heterogeneous cohort, it suggests a clinical value for accurate molecular profiling in a subset of poor prognosis paediatric patients.
- Van Tilburg CM, et al. ASCO Virtual Meeting, 29-31 May 2020,Abstract LBA10503.
Posted on
Previous Article
« VIALE-A: newly diagnosed chemo-ineligible AML Next Article
Letter from the Editor »
« VIALE-A: newly diagnosed chemo-ineligible AML Next Article
Letter from the Editor »
Table of Contents: ASCO 2020
Featured articles
COVID-19 & Telemedicine
COVID-19 and Cancer Consortium Registry: initial results
Oncology hospital-at-home model reduces hospitalizations, emergency department visits, and costs
Nurse-led telephone triage system reduces hospitalizations, helps patients manage symptoms at home
Melanoma
Adjuvant pembrolizumab: durable RFS for stage III melanoma
Adjuvant pembrolizumab: durable RFS for stage III melanoma
Pembrolizumab plus low-dose ipilimumab well tolerated after progression on PD1 antibody therapy
Toripalimab plus axitinib effective in metastatic mucosal melanoma
Breast & Ovarian Cancer
Advanced breast cancer: locoregional therapy does not improve OS
T-DM1 does not improve safety or efficacy in HER-2 positive early breast cancer; favorable iDFS reported
Maintenance olaparib improves OS in relapsed ovarian cancer with BRCA1/2 mutation
Combination pembrolizumab/chemo improves PFS in metastatic TNBC
Effect of veliparib with or without cisplatin in breast cancer: results of SWOG S1416
PHOEBE, a phase 3 trial comparing pyrotinib and lapatinib in HER2-positive metastatic breast cancer
BYLieve demonstrates efficacy of PIK3CA-directed treatment post CDK4/6-ihibition
Strategies emerge for chemotherapy de-escalation in HER2-positive breast cancer
Multiple Myeloma
Carfilzomib: no PFS benefit for multiple myeloma
Lung Cancer
ES-SCLC: tremelimumab + durvalumab + chemotherapy misses endpoint
Adjuvant osimertinib in NSCLC: practice changing ADAURA trial
ES-SCLC: pembrolizumab KEYNOTE-604 data
Second-line gemcitabine plus ramucirumab significantly improves overall survival
Tiragolumab and atezolizumab: ORR in NSCLC
MET-amplified advanced NSCLC responds well to MET inhibitor capmatinib
Genitourinary Cancer
Urothelial cancer: avelumab works as maintenance therapy
ARAMIS final OS and nmCRPC safety outcomes
Final survival results from phase 3 SPARTAN trial
Novel drug for kidney cancers/VHL patients
Primary analysis from IMvigor010, adjuvant atezolizumab in high risk muscle-invasive urothelial carcinoma
First randomised trial of Lu-PSMA in mCRPC progressing after docetaxel
Gastrointestinal Cancer
HER2-expressing metastatic colorectal cancer: trastuzumab deruxtecan
REGOMUNE: a phase 2 study combining regorafenib and avelumab
Cardiotoxicity: consider switching to S-1
Perioperative chemotherapy for resectable pancreatic ductal adenocarcinoma
Real-world data of sequential sorafenib followed by regorafenib in unresectable HCC
Paediatric Cancer
Sustained improvements in quality of life with larotrectinib
Promising first immunotherapy trial in placental trophoblastic tumours
Precision medicine for poor-prognosis paediatric patients
Related Articles
September 9, 2020
Sustained improvements in quality of life with larotrectinib
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

