https://doi.org/10.55788/5a690e4d
CtDNA is tumour DNA that can be detected in the bloodstream. Both, pre- and post-resection DNA has been associated with poor clinical outcomes [1].
Recently, results from the phase 3 CheckMate 915 trial (NCT03068455) showed that adjuvant dual immune checkpoint inhibition with nivolumab plus ipilimumab did not improve survival in patients with completely resected stage IIIC/D–IV melanoma compared with nivolumab alone [2]. Prof. Georgina Long (University of Sydney, Australia) presented the results of her current analysis of the predictive value of pre-treatment ctDNA for disease recurrence and survival in the adjuvant immunotherapy populations of the CheckMate 915 trial [3].
CheckMate-915 randomised 1,844 patients with resected stage IIIB/D–IV 1:1 to receive adjuvant nivolumab or nivolumab plus ipilimumab. Pre-treatment ctDNA was available from 1,127 patients (61% of intention-to-treat [ITT] population) who showed no difference in baseline characteristics and efficacy from the ITT population. A trend towards a greater prevalence of ctDNA-positivity in higher stage III substages of melanoma was observed.
Additionally, an association was observed between ctDNA positivity and recurrence-free survival (HR 1.87) and distant metastases-free survival (HR 2.86) during follow-up (see Figure). This association was particularly strong during the first months of treatment. Of note, no significant interaction between baseline ctDNA status and the treatment arm was observed.
The predictive value of baseline ctDNA improved when it was combined with baseline IFNγ-status and tumour mutational burden. Again, the association of this combined marker with early recurrence was highest in the first months of treatment.
Based on these results, Prof. Long concluded that “pre-treatment ctDNA was associated with an increased risk of early recurrence across treatment arms. ctDNA is a useful biomarker for combined analyses predicting outcome for adjuvant melanoma.”
- Lee B, et al. Ann Oncol 2019;30(9):1472–1478.
- Long GV, et al. Cancer Res. 2021;81(13_Supplement):CT004.
- Long GV, et al. Association of pre-treatment ctDNA with disease recurrence and clinical and translational factors in patients with stage IIIB-D/IV melanoma treated with adjuvant immunotherapy (CheckMate 915). Abstract 788O, ESMO Congress 2022, 09–13 September, Paris, France.
Figure: Association of baseline ctDNA with recurrence [3].
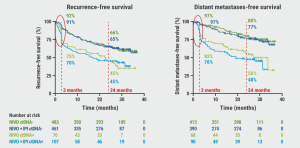
NIVO, nivolumab. IPI, ipilimumab.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« A pathway from air pollution to lung cancer in non-smokers identified Next Article
Survival-benefit of neoadjuvant T-VEC maintained over 5 years of follow-up »
« A pathway from air pollution to lung cancer in non-smokers identified Next Article
Survival-benefit of neoadjuvant T-VEC maintained over 5 years of follow-up »
Table of Contents: ESMO 2022
Featured articles
Letter from the Editor
Colorectal Cancer
High pathological responses to neoadjuvant immune checkpoint inhibition in locally advanced dMMR colon cancer
Fruquintinib: a potential new treatment for patients with refractory mCRC
Second-line avelumab is effective in patients with MSI-H/dMMR mCRC
Upper Gastrointestinal Cancer
Deep learning models predict the risk of relapse and the mutational profile in GIST
Addition of pembrolizumab to lenvatinib does not improve OS in advanced HCC
New, highly selective inhibitor of FGFR2 driver alterations and resistance mutations
Chemo-immunotherapy in gastric cancer is more effective when administered in parallel
Breast Cancer
Tumour infiltrating lymphocytes identify patients with immunogenic triple-negative breast cancer
OS benefit of abemaciclib in HR-positive/HER2-negative advanced breast cancer not (yet) statistically significant
OS benefit of sacituzumab govitecan in pre-treated HR-positive/HER2-negative metastatic breast cancer
Lung Cancer
A pathway from air pollution to lung cancer in non-smokers identified
Selective KRASG12C inhibitor sotorasib demonstrates superior PFS and ORR compared to docetaxel in previously treated patients with NSCLC
Promising clinical activity of tepotinib plus osimertinib in NSCLC with MET amplification after progression on first-line osimertinib
High pathological responses in borderline resectable NSCLC patients after induction with dual immunotherapy and concurrent chemoradiotherapy
Melanoma
Treatment with tumour-infiltrating lymphocytes for advanced melanoma outperforms ipilimumab
Neoadjuvant pembrolizumab outperforms adjuvant pembrolizumab in resectable stage III–IV melanomas
Survival-benefit of neoadjuvant T-VEC maintained over 5 years of follow-up
Baseline ctDNA predicts survival in resected stage III–IV melanoma
Genitourinary Cancer – Prostate Cancer
Overall survival benefit of abiraterone in mHSPC is maintained for 7 years
Limited benefit of adding long-term ADT to post-operative radiotherapy in prostate cancer
Intensified ADT benefits biochemical progression-free survival in biochemically relapsed prostate cancer
Genitourinary Cancer – Non-Prostate Cancer
Adjuvant nivolumab plus ipilimumab does not improve survival in patients with localised RCC at high risk of relapse after nephrectomy
Triple therapy improves progression-free survival in patients with advanced RCC versus dual therapy
Adjuvant atezolizumab does not improve outcomes for patients with RCC and increased risk of recurrence
Gynaecological cancers
OS benefit for advanced ovarian cancer patients treated with maintenance olaparib
Maintenance tegafur-uracil does not improve survival in locally advanced cervical cancer
Head and Neck Cancer
Adding first-line pembrolizumab to CRT in locally advanced HNSCC does not significantly prolong survival or event-free survival
5-FU-free chemotherapy combination as an alternative for first-line treatment of recurrent or metastatic HNSCC
Epstein Barr virus-specific autologous cytotoxic T lymphocytes do not improve survival in nasopharyngeal carcinoma
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

