Patients with cancer have an increased risk of complications from SARS-CoV-2 infection [1]. Vaccination is recommended, but the impact of chemotherapy and immunotherapy on immunogenicity and safety is still unclear [2]. The prospective, multicentre, non-inferiority VOICE trial (NCT04715438) aimed to assess the impact of immunotherapy, chemotherapy, and chemo-immunotherapy on immunogenicity and safety of SARS-CoV-2 vaccination in patients treated for a solid tumour [3].
VOICE compared 4 cohorts: individuals without cancer (A) and patients with solid tumours who were treated with immunotherapy (B), chemotherapy (C), or chemo-immunotherapy (D). Participants received 2 mRNA-1273 vaccinations 28 days apart. The primary endpoint was SARS-CoV-2 Spike S1-specific IgG serum antibody response, defined as >10 binding antibody units (BAU)/mL, 28 days after the second vaccination. An adequate response was defined as a cut-off level of 300 BAU/mL based on the neutralising capacity of vaccine-induced antibodies. First results were presented by Dr Sjoukje Oosting (University Medical Center Groningen, The Netherlands).
In total, 743 of 791 enrolled participants were antibody-negative at baseline, received 2 mRNA-1273 vaccinations 28 days apart, and completed assessments on day 28 after the second vaccination (per protocol population). The primary endpoint was achieved in 100%, 99.3%, 97.4%, and 100% of the participants in cohorts A, B, C, and D. The antibody response was considered adequate after the second vaccination in respectively 99.6%, 93.1%, 83.8%, and 88.8% in cohorts A, B, C, and D. Moreover, spike-specific T cell responses were detected in 46.7% of suboptimal and non-responders. No new safety signals were observed.
“These data show that vaccination with mRNA-1273 is safe in patients receiving immunotherapy, chemotherapy, or chemo-immunotherapy for a solid tumour,” concluded Dr Oosting. “Seroconversion rate is very high after 2 vaccinations and non-inferior to controls. However, a significant minority of patients does not develop an adequate antibody response. An additional booster may turn inadequate into adequate responders. In addition, longer follow-up will indicate if there is a difference in the duration of immune response between patients and controls.”
In line with these results, results from CAPTURE (NCT03226886), presented by Dr Scott Shepherd (Royal Marsden NHS Foundation Trust, UK) [4], showed that infection with SARS-CoV-2 induces durable immune responses in cancer patients. However, haematological malignancy patients had impaired immune responses that were disease and treatment-specific (anti-CD20), but with evidence suggestive of compensation from T cells. In addition, neutralising responses to Beta and Delta variants are reduced when compared with wildtype SARS-CoV-2. CAPTURE also showed a diminished serological response to vaccination in patients with haematological malignancies, in particular a diminished serological response to Delta variant. However, prior SARS-CoV-2 infection booster vaccines induced responses. “These results lend support to prioritisation of all cancer patients for further booster vaccination,” concluded Dr Shepherd.
- Venkatesulu BP, et al. JNCI Cancer Spectr. 2021; 5(2):pkaa102.
- Garassino MC, et al. Ann Oncol. 2021;32:579–581.
- Oosting S, et al. Vaccination against SARS-CoV-2 in patients receiving chemotherapy, immunotherapy, or chemo-immunotherapy for solid tumors. Abstract LBA8, ESMO Congress 2021, 16–21 September.
- Shepherd STC, et al. Adaptive immunity to SARS-CoV-2 infection and vaccination in cancer patients: The CAPTURE Study. Abstract 1557O, ESMO Congress 2021, 16–21 September.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Cancer patients more likely to die from COVID-19 when hospital admittance is required Next Article
Low numbers of M2 macrophages in tumour microenvironment associated with superior response to immunotherapy in Hodgkin lymphoma »
« Cancer patients more likely to die from COVID-19 when hospital admittance is required Next Article
Low numbers of M2 macrophages in tumour microenvironment associated with superior response to immunotherapy in Hodgkin lymphoma »
Table of Contents: ESMO 2021
Featured articles
Breast Cancer
Trastuzumab deruxtecan triples PFS
Novel conjugate meets primary endpoint
Longest survival benefit from first-line CDK4/6 inhibitor
Meta-analysis shows 6-months adjuvant trastuzumab is optimal
Double-positive results for triple-negative metastatic breast cancer
Survival after neoadjuvant therapy with trastuzumab-lapatinib plus chemotherapy
Postmenopausal breast cancer: extended letrozole reduces recurrence
Asian women also benefit from palbociclib plus letrozole
No PEARLs of survival with palbociclib plus endocrine therapy compared with capecitabine, but QoL better
Gastrointestinal Cancer
Neoadjuvant chemotherapy potential alternative to neoadjuvant chemoradiotherapy in LARC
Immune chemo-sensitisation looks promising in microsatellite-stable mCRC
Adagrasib shows promising clinical activity in heavily pretreated KRAS-mutated CRC
Automated detection of microsatellite status on unstained samples in early colon cancer
Consistent benefit of anti-PD-1 therapy for oesophageal and gastric cancer
HIPEC in gastric cancer with peritoneal metastases
ctDNA highly predictive in HER2-positive, advanced gastric or gastro-oesophageal junction cancer
Lung Cancer
Robust anticancer activity of trastuzumab deruxtecan in HER2-mutated NSCLC
Nivolumab/ipilimumab continues to provide survival benefit in unresectable MPM
Adjuvant atezolizumab lowers relapse rate in resected NSCLC
Three-year OS follow-up from CASPIAN trial
TCR clonality predicts pembrolizumab response in NSCLC
Melanoma
Adjuvant immunotherapy reduces risk of disease recurrence in stage II melanoma
IFN-γ signature predicts response to immunotherapy
Updated results of SECOMBIT trial
Combining T-VEC and pembrolizumab does not significantly improve survival in advanced, unresectable melanoma
Durable intracranial responses with nivolumab/ipilimumab
Genitourinary Cancer
TKI drug-free interval strategy not detrimental to conventional continuation strategy in RCC
Modified ipilimumab schedule reduces risk of grade 3/4 adverse events
Optimal neoadjuvant dose ipilimumab/nivolumab in stage III urothelial cancer
Better survival with neoadjuvant dose-dense MVAC regimen in MIBC
PARP inhibitor rechallenge improves PFS in ovarian cancer
Pembrolizumab prolongs survival in persistent, recurrent, or metastatic cervical cancer
Pembrolizumab has durable effect in previously treated MSI-H/dMMR advanced endometrial cancer
HRR mutational status is prognostic and predictive biomarker olaparib activity
Haematological Cancer
Mutational analyses are predictive in malignant lymphomas
Low numbers of M2 macrophages in tumour microenvironment associated with superior response to immunotherapy in Hodgkin lymphoma
COVID-19
Adequate response to SARS-CoV-2 vaccine in cancer patients
Cancer patients more likely to die from COVID-19 when hospital admittance is required
Third global survey of the ESMO Resilience Task Force
High COVID-19 mortality in Swiss cancer patients
Basic Science & Translational Research
Neutrophils negatively correlate with response to anti-PD-1 monotherapy in dMMR tumours
Tetraspecific ANKETs harnesses innate immunity in cancer therapies
Early ctDNA reduction in metastatic uveal melanoma correlates better with OS than RECIST response
Gut microbiota as a potential predictive biomarker
Related Articles
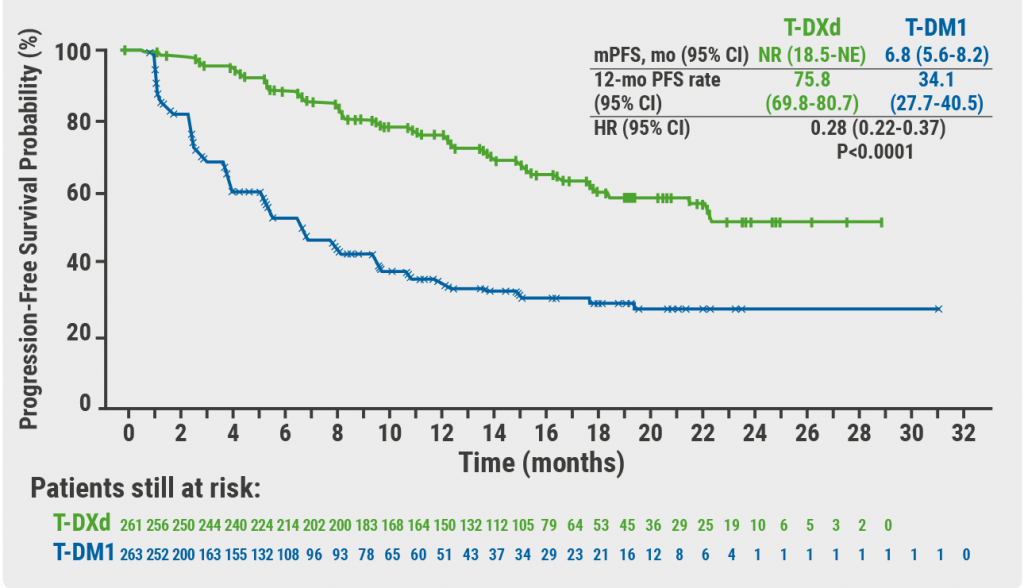
November 19, 2021
Trastuzumab deruxtecan triples PFS
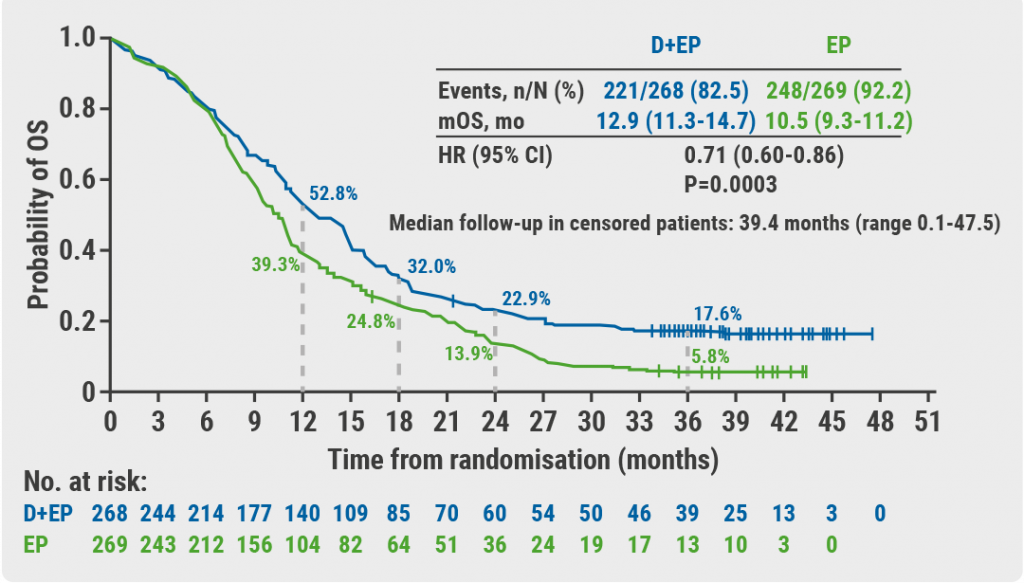
November 19, 2021
Three-year OS follow-up from CASPIAN trial
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

