Headaches are among the most frequent side effects after vaccination against SARS-CoV-2. “More than half of patients receiving vaccines report headaches,” said Dr Carl Göbel (Universitätsklinikum Schleswig-Holstein, Germany).
The incidence of headaches was 55.1% after the Pfizer/BioNTech vaccine (BNT162b2), 57.5% after the AstraZeneca vaccine (AZD1222), and 64.7% after the Moderna vaccine (mRNA-1273) [4–6]. “mRNA vaccines are used for the first time,” Dr Göbel added. “A new headache phenotype has appeared, but the precise phenotype has not yet been described.” The aim of the current study was to compare this phenotype with and differentiate it from other forms of primary and secondary headaches.
Dr Göbel and colleagues developed a survey that was available both offline and online in order to capture the clinical characteristics of headache after vaccination. They distributed the survey to 12,000 care homes for the elderly as well as university hospitals in Germany and the United Arab Emirates [1].
Across all vaccines, the mean latency was 31.9 hours after vaccination. The latency with BioNTech/Pfizer was 34.4 hours, which was significantly longer than the Moderna vaccine (30.5 hours; P=0.008) and the AstraZeneca vaccine (26.7 hours; P<0.001).
Headache intensities across all vaccines were most frequently described as moderate or severe, although a substantial proportion of patients reported very severe headaches (see Figure). Headache intensity was lowest after BioNTech/Pfizer vaccination (3.52 on a scale of 0–5), which was significantly less intense than after Moderna (3.66; P<0.001) or AstraZeneca (3.55; P=0.03).
The headaches lasted on average 18.4 hours. Duration was shortest after BioNTech/Pfizer (17.7 hours) compared with the AstraZeneca vaccine (19.2 hours; not significant) and the Moderna vaccine (20.8 hours; P=0.04).
Figure: Headache intensities of all vaccines, frequency distribution [1]
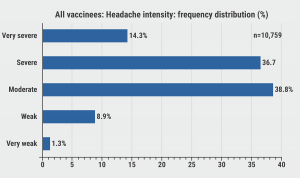
According to Dr Göbel, this data has an important clinical implication for differentiating between post-vaccine headaches and headaches due to a cerebral venous thrombosis as a complication of vaccination. “Headaches due to the latter usually did not start until 5 days after vaccination, while the post-vaccination headaches usually disappeared by day 5.”
- Göbel C, et al. Comparison of quantitative headache parameters of headache after vaccination against COVID-19 (Coronavirus SARS-CoV-2) with AZD1222, BNT162b2, mRNA-1273 and BBIBP-CorV vaccines. AL012, IHC 2021, 8–12 September.
- Göbel CH, et al. Brain Commun. 2021;3(3):fcab169.
- Göbel CH, et al. Pain Ther. 2021:1–22.
- European Medicines Agency (2021). COVID-19 vaccine AstraZeneca. Assessment report. Procedure No. EMEA/H/C/005675/0000, EMA/94907/2021:1-181.
- FDA Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. Vaccines and related biological products advisory committee meeting December 10, 2020.
- European Medicines Agency (2021). COVID-19 vaccine Moderna. Assessment report. Procedure No. EMEA/H/C/005791/0000, EMA/15689/2021 Corr.1:1–169.
Copyright ©2021 Medicom Medical Publishers[metaslider id=24100]
Posted on
Previous Article
« Grey matter cortical changes in patients with persistent headache after COVID-19 Next Article
Telemedicine beneficial for headache care during the pandemic »
« Grey matter cortical changes in patients with persistent headache after COVID-19 Next Article
Telemedicine beneficial for headache care during the pandemic »
Table of Contents: IHC 2021
Featured articles
Letter from the Editor
COVID-19
Telemedicine beneficial for headache care during the pandemic
Comparison of headaches after SARS-CoV-2 vaccination
Grey matter cortical changes in patients with persistent headache after COVID-19
Increased risk of cerebral venous thrombosis in COVID-19
Patient Perception and Symptoms
Predictors of health-related quality of life in cluster headache
Dry eye disease is more prevalent in migraine
Voice change and throat swelling are cranial autonomic symptoms in primary headache
Association between physical inactivity and headache disorders
Increased suicidal attempts and risks of ideation in medication-overuse headache
Cardioembolic Comorbidities
AI-enabled ECG algorithm predicts atrial fibrillation risk in migraine
Migraine may not be a risk factor for stroke
Imaging
Functional brainstem somatotopy of the trigeminal nerve during nociception
Morphological changes in cluster headache between attacks
Interictal pontine metabolism in migraine patients without aura
Genome-Wide Association Studies
Largest genome-wide association study of migraine to date
Robust evidence that cluster headache has a genetic basis
Pharmacological Treatment
Insights in drug-drug interactions facilitate rational polypharmacy
Rimegepant confers long-term improvements in MMDs
First real-world effectiveness data of erenumab is promising
Galcanezumab effective in patients with episodic or chronic cluster headache
Central effects and affected somatosensory processing with galcanezumab in migraine
Long-term safety and tolerability of atogepant in migraine
Non-Pharmacological Treatment
Occipital nerve stimulation effective and safe in chronic cluster headache
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

