Galcanezumab is a monoclonal antibody (mAb) that binds calcitonin gene-related peptide (CGRP) and prevents its biological activity without blocking the CGRP receptor. The phase 3b, open-label safety CGAR study (NCT02797951) evaluated the safety of galcanezumab within the context of expected medical practice in patients (n=163) with episodic or chronic cluster headache. Dr Charly Gaul (Headache Clinic Königstein, Germany) presented a post hoc efficacy analysis from this study.
Using the Patient Global Impression of Improvement (PGI-I), 81% of patients reported improvement of their cluster headache condition as very much better, much better, or a little better at 1 month after starting galcanezumab; this was 91% at 6 months and 93% at 12 months (see Figure). Similarly, the mean PGI-I score was 2.4 at 1 month after starting galcanezumab, 2.0 at 6 months, and 1.8 at 12 months. Mean change in PGI-I was associated with the mean change in European Quality 5-Dimensions 5-Levels (EQ-5D-5L) scores by the first month after starting galcanezumab.
Figure: Patients-reported improvement of cluster headache using the PGI-I after treatment with galcanezumab [1]
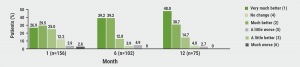
These data indicate an overall improvement in health status in patients with cluster headache in response to open-label galcanezumab treatment.
- Gaul C, et al. Efficacy Results from an Open-Label Safety Study of Galcanezumab in Patients with Episodic or Chronic Cluster Headache. AL076, IHC 2021, 8–12 September.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Central effects and affected somatosensory processing with galcanezumab in migraine Next Article
First real-world effectiveness data of erenumab is promising »
« Central effects and affected somatosensory processing with galcanezumab in migraine Next Article
First real-world effectiveness data of erenumab is promising »
Table of Contents: IHC 2021
Featured articles
Letter from the Editor
COVID-19
Telemedicine beneficial for headache care during the pandemic
Comparison of headaches after SARS-CoV-2 vaccination
Grey matter cortical changes in patients with persistent headache after COVID-19
Increased risk of cerebral venous thrombosis in COVID-19
Patient Perception and Symptoms
Predictors of health-related quality of life in cluster headache
Dry eye disease is more prevalent in migraine
Voice change and throat swelling are cranial autonomic symptoms in primary headache
Association between physical inactivity and headache disorders
Increased suicidal attempts and risks of ideation in medication-overuse headache
Cardioembolic Comorbidities
AI-enabled ECG algorithm predicts atrial fibrillation risk in migraine
Migraine may not be a risk factor for stroke
Imaging
Functional brainstem somatotopy of the trigeminal nerve during nociception
Morphological changes in cluster headache between attacks
Interictal pontine metabolism in migraine patients without aura
Genome-Wide Association Studies
Largest genome-wide association study of migraine to date
Robust evidence that cluster headache has a genetic basis
Pharmacological Treatment
Insights in drug-drug interactions facilitate rational polypharmacy
Rimegepant confers long-term improvements in MMDs
First real-world effectiveness data of erenumab is promising
Galcanezumab effective in patients with episodic or chronic cluster headache
Central effects and affected somatosensory processing with galcanezumab in migraine
Long-term safety and tolerability of atogepant in migraine
Non-Pharmacological Treatment
Occipital nerve stimulation effective and safe in chronic cluster headache
Related Articles
August 27, 2019
The role of neurogenic inflammation in migraine
July 30, 2019
Lasmiditan: rapid onset of efficacy in acute migraine
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

