In 2018, erenumab received marketing authorisation in Switzerland for the prevention of migraine in adults. Real-world data evaluating the effect of erenumab in a setting of routine medical care in Switzerland is limited. The observational SQUARE study (CAMG334ACH01) aimed to address this data gap.
SQUARE is a prospective, non-interventional study evaluating erenumab in a post-marketing setting in Switzerland [1]. Patients with prior treatment with erenumab or any calcitonin gene-related peptide (CGRP)-based therapy or recent use of investigational drugs were excluded. Patients were observed over a period of 24 months. Prof. Andreas R. Gantenbein (Zurzach Care, Switzerland) shared results on the real-world effectiveness of erenumab at month 6 compared to baseline.
A total of 172 adult patients were enrolled from 19 centres, both migraine care specialist centres and general neurologist centres. Most participants were women (84.9%). At baseline, patients had an average of 16.6 monthly migraine days (MMDs) and 11.6 monthly acute migraine-specific medication (AMSM) days. Almost 54% of patients fulfilled the criteria for episodic migraine versus 46% for chronic migraine.
Erenumab significantly reduced the Headache Impact Test (HIT)-6 score from baseline to month 3 and month 6 (both P<0.001) in both men and women and in patients with either episodic or chronic migraine (see Figure). Erenumab also significantly reduced the HIT-6 score in all groups with PPTFs from baseline to month 3 and month 6 (all P≤0.001). These results suggest improved quality of life. In line with these results, erenumab significantly reduced modified (monthly) migraine disability assessment test (mMIDAS) during this treatment period in both sexes (both P<0.001), indicating improvement in migraine-related disability. Comparable results were found for Impact of Migraine on Partners and Adolescent Children (IMPAC), which shows a decreased impact on spouses and children of patients.
Figure: Effect of erenumab on HIT-6 score by sex or by migraine type [1]
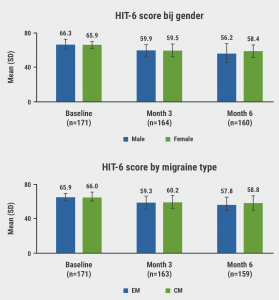
No difference was observed in treatment satisfaction between sexes, as measured by the Treatment Satisfaction Questionnaire for Medication (TSQM)-9. There was a significant difference between the episodic and chronic migraine groups in TSQM-9 scores for effectiveness (P=0.011), but not for convenience or global satisfaction. PPTF had no significant impact on any TSQM-9 score.
Erenumab significantly reduced mean MMDs from baseline to months 3 and 6, irrespective of sex or migraine subtype (all P<0.001) and in all PPTF groups from baseline to month 3 (P=0.013) and month 6 (P=0.014). MMD response rates were not significantly different between sexes except 100% response rate for month 6 (P=0.015). MMD response rates were not significantly different between migraine types. Finally, erenumab significantly reduced the mean AMSM days (triptans and/or ergot derivates) from baseline to month 3 and month 6 (both P<0.001).
SQUARE was the first study that prospectively collected real-world data on erenumab under routine medical care in Switzerland. In addition to meaningful clinical improvements, patients reported consistent treatment satisfaction with erenumab across sexes, migraine type, and PPTF group.
- Gantenbein A, et al. Real-world effectiveness data of erenumab-treated migraine patients in Switzerland: The SQUARE study. AL078, IHC 2021, 8–12 September.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Galcanezumab effective in patients with episodic or chronic cluster headache Next Article
Rimegepant confers long-term improvements in MMDs »
« Galcanezumab effective in patients with episodic or chronic cluster headache Next Article
Rimegepant confers long-term improvements in MMDs »
Table of Contents: IHC 2021
Featured articles
Letter from the Editor
COVID-19
Telemedicine beneficial for headache care during the pandemic
Comparison of headaches after SARS-CoV-2 vaccination
Grey matter cortical changes in patients with persistent headache after COVID-19
Increased risk of cerebral venous thrombosis in COVID-19
Patient Perception and Symptoms
Predictors of health-related quality of life in cluster headache
Dry eye disease is more prevalent in migraine
Voice change and throat swelling are cranial autonomic symptoms in primary headache
Association between physical inactivity and headache disorders
Increased suicidal attempts and risks of ideation in medication-overuse headache
Cardioembolic Comorbidities
AI-enabled ECG algorithm predicts atrial fibrillation risk in migraine
Migraine may not be a risk factor for stroke
Imaging
Functional brainstem somatotopy of the trigeminal nerve during nociception
Morphological changes in cluster headache between attacks
Interictal pontine metabolism in migraine patients without aura
Genome-Wide Association Studies
Largest genome-wide association study of migraine to date
Robust evidence that cluster headache has a genetic basis
Pharmacological Treatment
Insights in drug-drug interactions facilitate rational polypharmacy
Rimegepant confers long-term improvements in MMDs
First real-world effectiveness data of erenumab is promising
Galcanezumab effective in patients with episodic or chronic cluster headache
Central effects and affected somatosensory processing with galcanezumab in migraine
Long-term safety and tolerability of atogepant in migraine
Non-Pharmacological Treatment
Occipital nerve stimulation effective and safe in chronic cluster headache
Related Articles
August 18, 2021
Factors associated with decreased migraine attack risk
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

