https://doi.org/10.55788/21402cd9
Cilta-cel is a dual-binding B-cell maturation antigen (BCMA)-directed CAR T-cell therapy, which has demonstrated a median progression-free survival of approximately 3 years in patients with MM who had received at least 3 prior lines of therapy in the previous CARTITUDE-1 trial (NCT03548207) [1]. Progressing from there, Dr Hermann Einsele (University of Würzburg, Germany) presented the primary results of the current phase 3 CARTITUDE-4 trial (NCT04181827), which tested cilta-cel against standard of care in earlier lines of therapy. The trial randomised 419 participants with lenalidomide-refractory MM who had received 1-3 prior lines of therapy 1:1 to cilta-cel or to the standard of care therapy by physician’s choice (DPd or PVd) [2]. The primary outcome measure was progression-free survival (PFS).
After a median follow-up of 15.9 months, the primary endpoint was met: PFS was significantly improved in participants in the cilta-cel arm compared with standard of care (HR 0.26; 95% CI 0.18–0.38; P<0.0001, see Figure). The corresponding 12-month PFS rates were 76% and 49%. This result was consistent across pre-defined subgroups. Dr Einsele added: “Importantly, the data suggest that participants who had received only 1 prior line of therapy may benefit more from cilta-cel than participants having received 2 or 3 prior lines of therapy”. Overall response rates were 84.6% versus 67.3% and the complete response rate was 73.1% in the cilta-cel versus 21.8% in the standard of care arm, respectively. In an as-treated analysis, the complete response rate was even higher in the as-treated population (99.4% vs 86.4%).
Looking at safety, 85%–90% of the patients experienced neutropenia. These events were almost exclusively grade 3 or 4 events, but mostly resolved to low-grade events by day 30. The grade 3 or 4 infection rate was similar for the 2 arms, with 26.9% in the experimental arm and 24.5% in the control arm. Cytokine release syndrome was seen in 76.1% of the ‘as-treated with cilta-cel’ population, but only 2 cases were grade 3 or 4 events. Finally, neurotoxicity was reported in 20.5% of the participants, including 5 cases that were of grade 3 or 4.
Figure: Primary endpoint of progression-free survival from the CARTITUDE-4 trial [2]
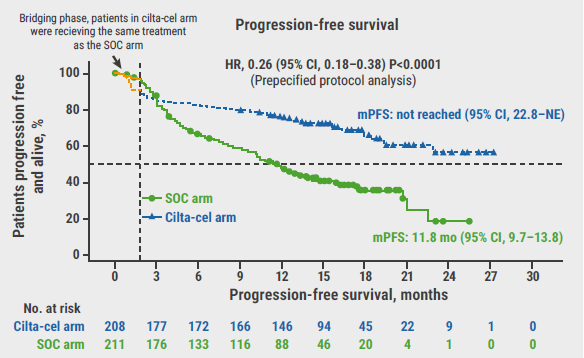
SOC, Standard of care; HR, hazard ratio; CI, confidence interval; mo, months; mPFS, mean progression-free survival; No., number; NE, not estimable.
- Berdeja JG, et al. Lancet. 2021;398(10297):314–324
- Einsele H, et al. Phase 3 results from CARTITUDE-4: Cilta-cel versus standard of care (PVd or DPd) in lenalidomide-refractory multiple myeloma. Plenary abstracts session, EHA 2023 Annual Congress, 8─11 June, Frankfurt, Germany.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Letter from the Editor Next Article
Encouraging results for low-dose belantamab mafodotin plus nirogacestat in patients with RRMM »
« Letter from the Editor Next Article
Encouraging results for low-dose belantamab mafodotin plus nirogacestat in patients with RRMM »
Table of Contents: EHA 2023
Featured articles
Multiple Myeloma
Can we combine teclistamab and nirogacestat for the treatment of RRMM?
Encouraging results for low-dose belantamab mafodotin plus nirogacestat in patients with RRMM
CARTITUDE-4: Cilta-cel meets expectations in lenalidomide-refractory MM
Lymphoma
Radiotherapy or not in patients with PMBCL after immunochemotherapy?
Durable responses for loncastuximab tesirine in relapsed/refractory DLBCL
Zandelisib promising in relapsed/refractory indolent B-cell NHL
Promising data for epcoritamab plus R-CHOP in untreated DLBCL
Non-Malignant Haematology
Investigational agent OMS906 performs well in PNH
Robust platelet responses with cevidoplenib in ITP
Leukaemia
QuANTUM-First: Updated results on quizartinib in AML with FLT3-ITD
Promising data for ziftomenib in relapsed/refractory NPM1-mutated AML
MRD-positive patients with FLT3-ITD AML may benefit from post-transplant gilteritinib
Deep responses with asciminib in CML-CP
QUIWI: First results suggest a clinical benefit of quizartinib in AML
Miscellaneous
COMMANDS trial: A paradigm shift in LR-MDS-associated anaemia
REVIVE: Rusfertide meets the primary endpoint in PV
Mapping healthy HPSC variations to diagnose haematopoietic abnormalities
High risk of death for individuals with C282Y/C282Y hereditary haemochromatosis and diabetes
Related Articles

December 22, 2021
PFGC 2021 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

