“The risk of relapse is high in patients with FLT3-ITD-mutated AML,” said Dr Mark Levis (Johns Hopkins Hospital, Maryland, USA). “Although FLT3 inhibitors are often administered as post-HCT maintenance therapy, the evidence for the usefulness of these agents in FLT3-ITD-mutated AML is inconclusive.” Dr Levis and colleagues investigated whether post-HCT maintenance therapy with gilteritinib, a potent FLT3 inhibitor, could provide a clinical benefit for patients with this form of AML. Additionally, they evaluated whether the tumour’s MRD status could facilitate the selection of patients who should receive post-HCT gilteritinib. The MORPHO trial (NCT02997202) randomised 356 participants 1:1 to 24 months of gilteritinib maintenance therapy or placebo post-HCT [1]. Relapse-free survival was the primary endpoint.
Considering the complete study population, the primary endpoint of relapse-free survival was not met, but a numerical benefit was observed for participants who were treated with gilteritinib (HR 0.68; 95% CI 0.46–1.01; P=0.052). Overall survival did not differ significantly between treatment groups (HR 0.85; P=0.44). MRD status at randomisation (thus pre-HCT) was, however, predictive of gilteritinib response: In the MRD-positive subset of participants, the primary endpoint was met (HR 0.52; 95% CI 0.32–0.84; P=0.0065, see Figure).
“We did not observe an increase in acute graft-versus-host-disease (GVHD), but chronic GVHD appeared to be more common in the gilteritinib arm than in the placebo arm (52.2% vs 42.4%),” explained Dr Levis. Drug-related adverse events of grade 3 or higher (61.2% vs 25.4%), and drug-related adverse events leading to treatment discontinuation (15.2% vs 7.9%) were more frequently observed in the experimental arm. Myelosuppression was the predominant cause of adverse events.
Overall, the authors concluded that post-HCT gilteritinib could be particularly beneficial for patients who are MRD-positive pre- or post-HCT.
Figure: Effect of detectable pre-HCT MRD on relapse-free survival per study arm [1]
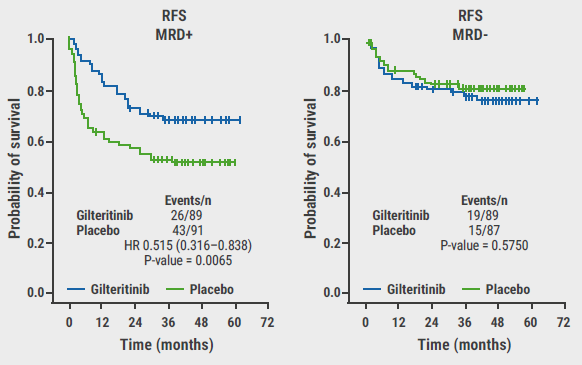
RFS, relapse-free survival; HR, hazard ratio.
- Levis M, et al. BMT-CTN 1506 (MORPHO): a randomized trial of the FLT3 inhibitor gilteritinib as post-transplant maintenance for FLT3-ITD AML. Late-breaking oral session, EHA 2023 Annual Congress, 8─11 June, Frankfurt, Germany.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Deep responses with asciminib in CML-CP Next Article
Promising data for ziftomenib in relapsed/refractory NPM1-mutated AML »
« Deep responses with asciminib in CML-CP Next Article
Promising data for ziftomenib in relapsed/refractory NPM1-mutated AML »
Table of Contents: EHA 2023
Featured articles
Multiple Myeloma
Can we combine teclistamab and nirogacestat for the treatment of RRMM?
Encouraging results for low-dose belantamab mafodotin plus nirogacestat in patients with RRMM
CARTITUDE-4: Cilta-cel meets expectations in lenalidomide-refractory MM
Lymphoma
Radiotherapy or not in patients with PMBCL after immunochemotherapy?
Durable responses for loncastuximab tesirine in relapsed/refractory DLBCL
Zandelisib promising in relapsed/refractory indolent B-cell NHL
Promising data for epcoritamab plus R-CHOP in untreated DLBCL
Non-Malignant Haematology
Investigational agent OMS906 performs well in PNH
Robust platelet responses with cevidoplenib in ITP
Leukaemia
QuANTUM-First: Updated results on quizartinib in AML with FLT3-ITD
Promising data for ziftomenib in relapsed/refractory NPM1-mutated AML
MRD-positive patients with FLT3-ITD AML may benefit from post-transplant gilteritinib
Deep responses with asciminib in CML-CP
QUIWI: First results suggest a clinical benefit of quizartinib in AML
Miscellaneous
COMMANDS trial: A paradigm shift in LR-MDS-associated anaemia
REVIVE: Rusfertide meets the primary endpoint in PV
Mapping healthy HPSC variations to diagnose haematopoietic abnormalities
High risk of death for individuals with C282Y/C282Y hereditary haemochromatosis and diabetes
Related Articles
August 9, 2019
Obinutuzumab/polatuzumab in follicular lymphoma
February 20, 2023
Ibrutinib plus venetoclax displays long-term benefits in CLL
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

