https://doi.org/10.55788/b1e8991f
“Patients with DLBCL who relapse after stem cell transplantation or CAR T-cell therapy, or who are refractory to second-line therapy have a poor prognosis,” said Dr Paolo Caimi (Cleveland Clinic, Ohio, USA). “There is an unmet need for accessible therapies with manageable toxicity profiles that have displayed long-term disease control in patients with relapsed or refractory DLBCL.” The phase 2 LOTIS-2 trial (n=145, NCT03589469) investigated the anti-tumour activity of the anti-CD19 monoclonal antibody-drug conjugate (conjugated drug: loncastuximab tesirine) in participants with relapsed/refractory DLBCL in [1] and Dr Caimi presented the long-term efficacy and safety results of this trial [2].
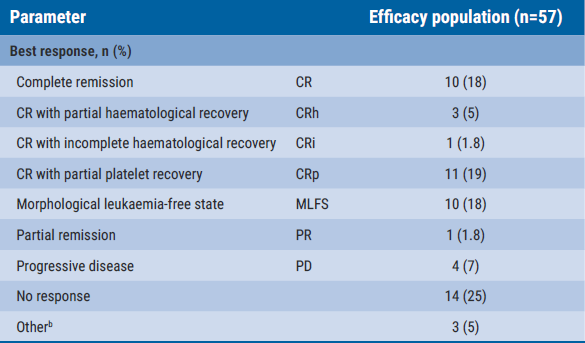
The overall response rate was 48.3% and the complete response rate was 24.8%. In the complete study population, the duration of response was 13.4 months, whereas the duration of response was ‘not reached’ in the subset of participants who had a complete response (n=36). The corresponding results for the median progression-free survival were 4.9 months and ‘not reached’. Also, the median overall survival was ‘not reached’ in the subset of participants who had a complete response and accumulated to 9.5 months in the overall study population.
According to the authors, no new safety signals emerged during the long-term follow-up. The most frequently reported grade ≥ 3 treatment-emergent adverse events were neutropenia (26%), thrombocytopenia (18%), increased gamma-glutamyl transferase (17%), and anaemia (10%).
Durable responses of loncastuximab tesirine were reported for participants with RR DLCBL during the long-term follow-up of the LOTIS-2 trial.
- Caimi PF, et al. Lancet. 2021;22(6):790─800
- Caimi PF, et al. Long-term responses with loncastuximab tesirine: updated results from LOTIS-2, the pivotal phase 2 study of patients with relapsed/refractory diffuse large B-cell lymphoma. P1132, EHA 2023 Annual Congress, 8─11 June, Frankfurt, Germany.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Zandelisib promising in relapsed/refractory indolent B-cell NHL Next Article
Radiotherapy or not in patients with PMBCL after immunochemotherapy? »
« Zandelisib promising in relapsed/refractory indolent B-cell NHL Next Article
Radiotherapy or not in patients with PMBCL after immunochemotherapy? »
Table of Contents: EHA 2023
Featured articles
Multiple Myeloma
Can we combine teclistamab and nirogacestat for the treatment of RRMM?
Encouraging results for low-dose belantamab mafodotin plus nirogacestat in patients with RRMM
CARTITUDE-4: Cilta-cel meets expectations in lenalidomide-refractory MM
Lymphoma
Radiotherapy or not in patients with PMBCL after immunochemotherapy?
Durable responses for loncastuximab tesirine in relapsed/refractory DLBCL
Zandelisib promising in relapsed/refractory indolent B-cell NHL
Promising data for epcoritamab plus R-CHOP in untreated DLBCL
Non-Malignant Haematology
Investigational agent OMS906 performs well in PNH
Robust platelet responses with cevidoplenib in ITP
Leukaemia
QuANTUM-First: Updated results on quizartinib in AML with FLT3-ITD
Promising data for ziftomenib in relapsed/refractory NPM1-mutated AML
MRD-positive patients with FLT3-ITD AML may benefit from post-transplant gilteritinib
Deep responses with asciminib in CML-CP
QUIWI: First results suggest a clinical benefit of quizartinib in AML
Miscellaneous
COMMANDS trial: A paradigm shift in LR-MDS-associated anaemia
REVIVE: Rusfertide meets the primary endpoint in PV
Mapping healthy HPSC variations to diagnose haematopoietic abnormalities
High risk of death for individuals with C282Y/C282Y hereditary haemochromatosis and diabetes
Related Articles
February 7, 2024
AUGMENT-101: Excellent results for revumenib in R/R KMT2Ar leukaemia
February 14, 2022
Clot-busting drugs appear safe in stroke patients taking blood thinners
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

