Dapagliflozin’s safety and efficacy was assessed in the DECLARE-TIMI 58 trial (n=17,160), which randomised patients 1:1 to either dapagliflozin 10 mg (n=8,582) or matching placebo (n=8,578). The mean age of the participants was 64, 37% was female, and the duration of follow-up was 4.2 years [1]. A distinguishing feature of DECLARE-TIMI 58 relative to other SGLT2i trials was the large population of patients without established cardiovascular disease (59.3%).
Dapagliflozin in peripheral artery disease patients
In 2018, dapagliflozin was shown to be superior to placebo in improving glycaemic control [1]. However, there is more to dapagliflozin than meets the eye, according to Prof. Marc P. Bonaca (University of Colorado, USA). Prof. Bonaca presented the outcomes of a subgroup analysis of peripheral artery disease (PAD) patients [3]. Out of the 17,160 DECLARE-TIMI-58 participants, 1,025 had lower extremity PAD. Of those, almost 75% were symptomatic mostly with claudication although 6% had critical limb ischaemia. Major adverse cardiac events (MACE) and renal events were higher among PAD patients in comparison with those without PAD, as were limb events. Event rates were higher among PAD patients, but researchers did not observe any effect modification by PAD status so absolute reductions in hospitalisation for heart failure and renal outcomes were greater in those with PAD. Overall, there was no difference with dapagliflozin vs placebo for major adverse limb events or amputation. A numerically higher rate of amputations was observed among PAD patients with dapagliflozin (8.4% vs 5.6%; HR 1.51, 95% CI, 0.94-2.42), but the difference was not statistically significant [2]. The rates for various limb events are shown in the Table.
Table: Outcomes limb events in peripheral artery disease patients (n=1,025) [2]
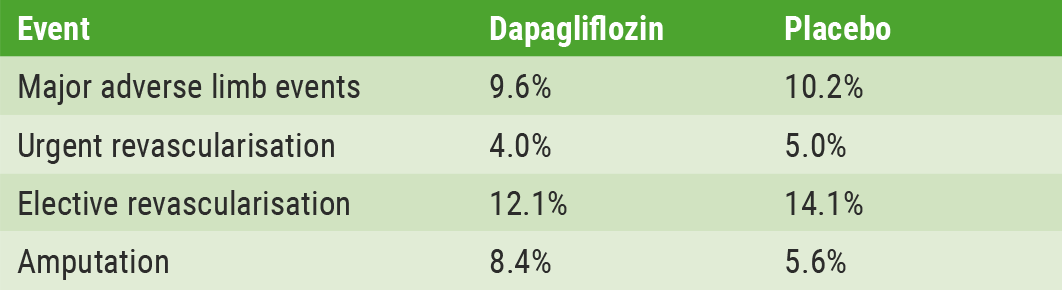
Major adverse limb events defined as acute limb ischaemia, critical limb ischaemia, amputation for ischaemia or urgent revascularisation for ischaemia.
Dapagliflozin in diabetes patients with heart failure or prior myocardial infarction subgroups
DECLARE subgroups analysis included, alongside patients with PAD, two other high-risk subgroups. One of those groups included patients with type 2 diabetes mellitus (DM2) and heart failure [3]. At baseline, 30% of randomised study participants had documented left ventricular ejection fraction; 3.9% of all 17,160 patients had HFrEF, and 7.7% had heart failure without known reduced ejection fraction. Dapagliflozin reduced heart failure hospitalisation and CV mortality across a wide spectrum of patients. Those benefitting the most were patients with HFrEF; they had a 38% reduction of death or heart failure hospitalisation compared with placebo, as well as a 45% reduction in CV death and 41% reduction in all-cause mortality with dapagliflozin vs placebo. Patients without HFrEF had a 12% reduction in CV mortality. Thus, according to the researchers, ejection fraction is a strong tool to identify patients who run the highest risk and for whom SGLT2 inhibitors may offer a particular robust benefit.
The third subgroup analysis was on DM2 patients with prior myocardial infarction (MI; n=3,584). The results showed that in these patients, dapagliflozin reduced the relative risk of MACE by 16% (percentage of patients with events 15.2% for dapagliflozin vs 17.8% for placebo; HR 0.84; 95% CI, 0.72-0.99; P=0.039) and the absolute risk by 2.6%. In patients without prior MI, there was no clear effect (7.1% vs 7.1%; HR 1.00; 95% CI, 0.88-1.13; P=0.97; P-interaction for relative difference 0.11) [4].
Overall, in the context of these subgroup analyses, Prof. Bonaca stated that these are important findings: “While the drug works the same for everyone with regard to relative risk reduction, these subgroups as mentioned above are at a higher risk of CV death and heart failure and renal complications. The absolute benefits of treatment with dapagliflozin for them are even greater. In general, when considering all high-risk patients, it is worth thinking about these drugs early including their use in primary prevention populations” [2].
1. Wiviott SD, et al. N Engl J Med 2019. Doi: 10.1056/NEJMoa1812389. Epub ahead of print.
2. Bonaca MP, et al. Abstract 413-14. ACC 2019, 16-18 March, New Orleans, USA.
3. Kato ET, et al. Circulation. 2019 Mar 18. Doi: 10.1161/CIRCULATIONAHA.119.040130. Epub ahead of print.
4. Furtado RHM, et al. Abstract 906-04. ACC 2019, 16-18 March, New Orleans, USA.
Posted on
Previous Article
« Letter from the Editor Next Article
Substantial impact of temporary interruptions of warfarin versus DOAC »
« Letter from the Editor Next Article
Substantial impact of temporary interruptions of warfarin versus DOAC »
Table of Contents: ACC 2019
Featured articles
Acute and Stable Ischaemic Heart Disease
Arrhythmias and Clinical Electrophysiology
Substantial impact of temporary interruptions of warfarin versus DOAC
Smartwatch can detect atrial fibrillation with high degree of accuracy
Congenital Heart Disease
Heart Failure and Cardiomyopathies
Frequent use of beta-blocker after HFpEF hospitalisations in elderly patients without compelling indications
High 5-year survival rates for older HF patients without initial severe comorbidity
Pulmonary Arterial Hypertension and Venous Thromboembolism
Interventional Cardiology
Vascular Medicine
Lower rates stroke/SE with DOACs in frail non-valvular AF patients
Similar rates of stroke/SE associated with DOAC vs warfarin use in obese non-valvular AF patients: Results from an observational registry
Convincing evidence of the role of icosapent in reducing subsequent CV events
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

