https://doi.org/10.55788/8d122648
Most patients with KMT2Ar acute leukaemia relapse after transplantation and there are no approved therapies for patients with this condition. Also, the menin-KMT2A interaction is an important driver of leukaemogenesis [1].
In the phase 2 AUGMENT-101 trial (NCT04065399), the oral menin inhibitor revumenib was assessed in paediatric and adult participants with R/R KMT2Ar acute leukaemia for efficacy (n=57) and safety (n=94) [2]. Approximately a quarter of the participants were below 18 years of age. Enrolled participants received revumenib (163 mg or 95 mg/m2 in case a participant’s body weight was below 40 kg) every 12 hours plus a strong CYP3A4 inhibitor in 28-day cycles. The primary endpoint was the complete remission (CR) rate plus CR with partial haematological recovery (CRh). The key findings were presented by Dr Ibrahim Aldoss (City of Hope National Medical Center, CA, USA).
The overall response rate was 63% (see Table) and after a follow-up of 6.1 months, 13 participants achieved CR plus CRh, meeting the primary endpoint threshold of 10% (22.8%; 95% CI 12.7–35.8; P=0.0036). The composite CRc rate (defined as CR+CRh+CR with incomplete platelet recovery+CR with incomplete count recovery) was 44% and 68% of participants who achieved CRc reached minimal residual disease (MRD)-negativity.
Table: Best response results from the AUGMENT-101 trial [2]
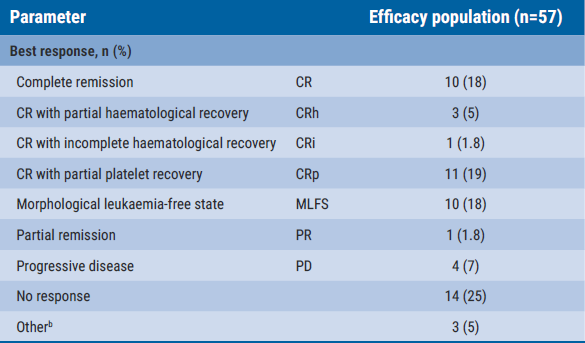
bIncludes patients without post-baseline disease assessment
The most frequently observed grade ≥3 treatment-emergent adverse events were differentiation syndrome (16.0%), febrile neutropenia (37.2%), and QTc prolongation (13.8%). “No participants discontinued due to differentiation syndrome, QTc prolongation, or cytopenia,” added Dr Aldoss.
“Based on these data, a new drug application for KMT2Ar leukaemia has been initiated under the FDA real-time oncology review programme,” Dr Aldoss announced in his talk.
- Issa GC, et al. Blood Cancer J. 2021;11(9):162.
- Aldoss I, et al. Revumenib Monotherapy in Patients with Relapsed/Refractory KMT2Ar Acute Leukemia: Topline Efficacy and Safety Results from the Pivotal Augment-101 Phase 2 Study. Abstract LBA-5, 65th ASH Annual Meeting, 9–12 December 2023, San Diego, CA, USA.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Blinatumomab reduces toxicity in the consolidation phase in paediatric high-risk B-cell ALL Next Article
Promising results for quizartinib, venetoclax, and decitabine in FLT3-ITD mutated AML »
« Blinatumomab reduces toxicity in the consolidation phase in paediatric high-risk B-cell ALL Next Article
Promising results for quizartinib, venetoclax, and decitabine in FLT3-ITD mutated AML »
Table of Contents: ASH 2023
Featured articles
Meet the Trialist: Prof. Jeff Sharman on ELEVATE-TN
Leukaemia
FLT3-ITD-specific MRD assessment useful for clinical management of AML
MRD status rather than FLT3-ITD co-mutation is linked to the benefit of CR1-allo in NPM1-mutated AML
Promising results for quizartinib, venetoclax, and decitabine in FLT3-ITD mutated AML
AUGMENT-101: Excellent results for revumenib in R/R KMT2Ar leukaemia
Blinatumomab reduces toxicity in the consolidation phase in paediatric high-risk B-cell ALL
Promising results for olverembatinib in combination with venetoclax for Ph+ ALL
Undetectable MRD on maintenance venetoclax, acalabrutinib, and obinutuzumab in the majority of R/R CLL participants
Lymphoma
Is allogeneic stem cell transplantation a solid option in R/R LBCL or R/R T-cell lymphoma?
Encouraging results for the addition of acalabrutinib to lenalidomide and rituximab in follicular lymphoma
Can ibrutinib ameliorate outcomes in R/R ABC-DLBCL undergoing autoSCT?
Primary phase 2 efficacy and safety results of M-Pola in relapsed/refractory LBCL
SYMPATICO: Ibrutinib plus venetoclax boosts PFS in R/R mantle cell lymphoma
Multiple Myeloma
KdD outperforms Kd in R/R MM also in participants with poor renal function
IsKia: Novel treatment regimen for MM delivers high MRD-negativity rates
Novel standard-of-care in newly diagnosed MM
Myeloproliferative Neoplasms
TRANSFORM-1: High spleen volume reduction rates for navitoclax plus ruxolitinib in myelofibrosis
Momelotinib beats controls regarding transfusion outcomes in myelofibrosis
DALIAH: Peginterferon-α head-to-head against hydroxyurea in MPN
Non-Malignant Haematology
Long-term efficacy and safety of iptacopan in PNH with anaemia
ADVANCE IV: Swift responses on efgartigimod in ITP
Favourable QoL and bleeding outcomes for rilzabrutinib in ITP
Novel risk assessment model acts on increasing hospital-acquired venous thromboembolism rates among children
Miscellaneous Topics
Axatilimab may present a new therapeutic strategy in chronic GvHD
Pomalidomide may become the first approved therapy for hereditary haemorrhagic telangiectasia
Ancestry-specific study into CH delivers new leads
Featured Interviews
Interview: Sandwich treatment model shows promise for mantle cell lymphoma
Meet the Trialist: Prof. Jeff Sharman on ELEVATE-TN
Related Articles
December 10, 2024
Menin inhibitors on the rise in KMT2Ar acute leukaemia
November 25, 2020
Tyrosine kinase inhibitor discontinuation can improve CML outcomes
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

