Some patients with ITP display partial response or no response to currently approved treatment protocols. Dr Catherine Broome (MedStar Georgetown University Hospital, Washington DC, USA) and colleagues hypothesised that a primary mechanism of thrombocytopenia in a subset of patients with ITP is classical complement pathway-dependent and that inhibition with sutimlimab would improve thrombocytopenia. Sutimlimab is a first-in-class humanised monoclonal antibody that selectively inhibits activation of the complement pathway by binding to complement protein 1 (C1).
An open-label phase 1b study (NCT03275454) included 12 patients with chronic ITP, many of those previously received a thrombopoietin receptor agonist (TPO-RA), rituximab, and splenectomy. In the first part of the trial, participants received sutimlimab on days 0 and 7, after which they received the medication biweekly for a maximum of 21 weeks, followed by a 9-week washout phase. If a clinically meaningful response was elicited from the first part of the trial, patients were included in a long-term extension phase.
Interim data demonstrated that sutimlimab resulted in a rapid and sustained response, with mean platelet count increasing to >50 × 109/L by day 1. This response was maintained for the duration of the treatment. Overall, 5 patients achieved durable response with sutimlimab as monotherapy; 4 patients had to be removed because they either needed rescue therapy or were unresponsive to sutimlimab.
Washout kinetics demonstrated that thrombocytopenia reoccurred when sutimlimab was discontinued and resolved upon retreatment in the long-term extension phase, corroborating the observed clinical effects of classical complement pathway inhibition. The therapeutic effect of sutimlimab was sustained during the long-term extension to week 79.
- Broome CM, et al. Long-Term Safety and Efficacy of Sutimlimab in Patients with Chronic Immune Thrombocytopenia. 62nd ASH Annual Meeting, 5-8 December 2020. Abstract 23.
Posted on
Previous Article
« BTK inhibition provides clinically active and durable platelet response Next Article
Mycophenolate efficacious and tolerable, even in elderly patients »
« BTK inhibition provides clinically active and durable platelet response Next Article
Mycophenolate efficacious and tolerable, even in elderly patients »
Table of Contents: ASH 2020
Featured articles
COVID-19
More complicated course of COVID-19 in leukaemia patients
Older age and imatinib treatment associated with COVID-19 mortality in CML
Allogeneic SARS-CoV-2-specific T cells to treat COVID-19
More severe COVID-19 outcomes for patients with haematologic malignancies
Acute Lymphoblastic Leukaemia
Improved outcomes, but still substantial part experiences relapses
Strong correlation between peripheral blood and bone marrow NGS MRD
Encouraging outcomes after autoHCT in patients with ALL
Acute Myeloid Leukaemia
Prognostic validity of AML composite model in predicting mortality
Venetoclax plus hypomethylating agents in favourable-risk AML
Encouraging clinical activity of decitabine plus ipilimumab in R/R or secondary MDS/AML
AML patients with specific mutations are unlikely to achieve MRD
Comparable outcomes with gilteritinib or quizartinib in R/R AML
First-in-class macrophage immune checkpoint inhibitor in AML
Bispecific DART® as salvage therapy for primary induction failure and early relapse
Gilteritinib in R/R AML patients priorly treated with midostaurin or sorafenib
Addition of venetoclax provides an effective, lower-intensity regimen
Chronic Leukaemia
Bosutinib effective and well tolerated in newly diagnosed CP-CML
Efficacy and safety of ponatinib in patients with CP-CML who failed second-generation TKIs
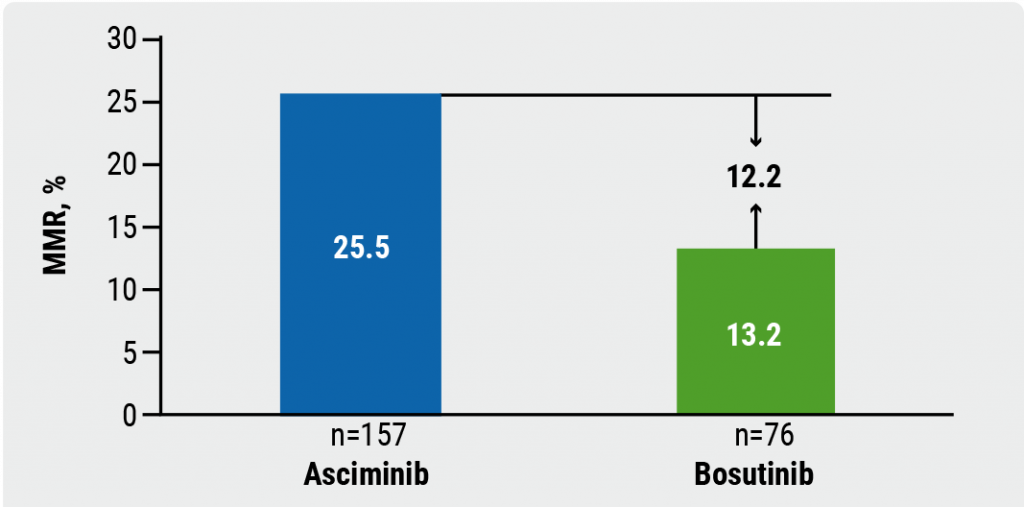
First-in-class STAMP inhibitor versus bosutinib in resistant or intolerant CML
PFS and ORR benefits of first-line ibrutinib-based treatment in CLL
Multiple Myeloma
Validation of MY-RADS response assessment category criteria
High symptom burden in transplant-ineligible patients with newly diagnosed MM
Added value of ixazomib to lenalidomide plus dexamethasone in transplant-ineligible newly diagnosed MM
Survival of transplant-eligible newly diagnosed MM in FORTE trial
Better survival with upfront autoSCT versus bortezomib-based intensification
Subcutaneous daratumumab plus pomalidomide and dexamethasone in R/R MM
Melflufen well tolerated with encouraging activity in heavily pretreated R/R MM
Initial data of FcRH5/CD3 T-cell-engaging bispecific antibody
Lymphoma
CD58 aberrations limit durable responses to CD19 CAR T-cell therapy
Anti-CD19 CAR T-cell therapy in relapsed/refractory indolent NHL
Myeloproliferative Neoplasms
MPN disease burden, quality of life, and treatment patterns
Interventions in JAK/STAT signalling pathway
Novel, orally available inhibitor of BCL-XL/BCL-2
New insights into genetics of MPN
Immune Thrombocytopenia
Mycophenolate efficacious and tolerable, even in elderly patients
First-in-class antibody sutimlimab selectively inhibits classical complement pathway
BTK inhibition provides clinically active and durable platelet response
Haemophilia, Sickle Cell Disease, Thalassaemia
First results from gene therapy trial in haemophilia B
Impact of haemophilia on children and their caregivers
Promising CRISPR gene editing results in β-thalassaemia and sickle cell disease
Erythroid maturation agent in patients with β-thalassaemia requiring regular RBC transfusions
Related Articles
February 18, 2021
First-in-class macrophage immune checkpoint inhibitor in AML
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

