The IL-17 family of cytokines is important in the regulation of both innate and adaptive host defences and inflammation. Not only IL-17A but also IL-17F is expressed in psoriasis lesional skin and amplifies tissue damage and inflammation. Bimekizumab neutralises the biologic function of both IL-17F and IL-17A, which might have more benefits than the sole blockade of IL-17A. “We have interesting data to suggest that IL-17F contributes to the psoriasis disease process, so hopefully with this drug we will see a higher response,” said Prof. Reich (University Medical Center Hamburg-Eppendorf, Germany).
BE VIVID
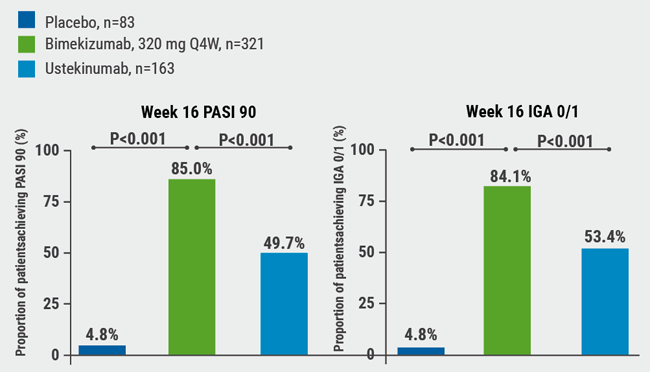
In the BE VIVID trial, 567 patients with moderate-to-severe psoriasis were randomised to bimekizumab (320 mg every 4 weeks), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (every 4 weeks through week 16, then bimekizumab 320 mg every 4 weeks) [1]. Co-primary endpoints were a Psoriasis Area and Severity Index (PASI) ≥90 and an Investigator’s Global Assessment (IGA) response of 0 or 1 (clear or almost clear skin).
At week 16, 85.0% and 84.1% of the patients in the bimekizumab arm compared with 49.7% and 53.4% of patients treated with ustekinumab and 4.8% each in the placebo arms achieved PASI 90 and IGA 0/1 (P<0.001 for all comparisons) (see Figure). In the bimekizumab arm, 58.6% of patients achieved the secondary endpoint of complete skin clearance (PASI 100), compared with 20.9% of patients in the ustekinumab arm and none of the placebo patients.
Figure: BE VIVID PASI 90 and IGA 0/1 at week 16 [1]

IGA, Investigator’s Global Assessment; PASI, Psoriasis Area and Severity Index; Q4W, every 4 weeks.
Treatment effects were maintained over time: at week 52, patients treated with bimekizumab achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% in the ustekinumab arm.
Over the year, 6.1% of patients treated with bimekizumab and 7.4% of patients treated with ustekinumab experienced serious adverse events (AEs). The most common reported AEs in the bimekizumab arm were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%). Four patients died, which was considered unrelated to treatment. There were no new or unexpected side effects.
To conclude, this first phase 3 data indicates that by inhibiting IL-17F in addition to IL-17A, the clinical efficacy increases. The agent not only has a very fast onset of action but also a very stable response over the 52-week period.
BE READY
The BE READY study was a second study assessing the safety and efficacy of bimekizumab, presented by Prof. Kenneth B. Gordon (Medical College of Wisconsin, USA) [2]. Participants (n=435) with moderate-to-severe psoriasis were treated with 320 mg bimekizumab or placebo and followed for an initial 16 weeks. Patients who achieved PASI ≥90 at week 16 were re-randomised to receive continuous bimekizumab at 2 different dosing regimens: either 320 mg every 4 weeks [Q4W], 320 mg every 8 weeks [Q8W], or placebo through week 56. Relapse was defined as a PASI <75 from week 20.
At week 16, 90.8% and 92.6% of patients treated with the IL-17A/F blocker achieved a PASI 90 response and an IGA of 0/1 compared with 1.2% of patients in the placebo group (P<0.001).
Both patients treated continuously with 320 mg bimekizumab Q4W and those treated with 320 mg bimekizumab Q8W were able to maintain their PASI 90 response (86.8% and 91%, respectively) compared with 16.2% of patients who were treated with placebo. Median time to relapse was 28 weeks.
Similar to the BE VIVID trial, the most frequently reported AEs with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs 8% Q8W). The incidence of serious treatment-emergent AEs with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
According to Prof. Gordon, bimekizumab was generally well tolerated with no unexpected safety findings.
- Reich K, et al. Late-breaking abstract, AAD Virtual Meeting Experience, 12-14 June 2020.
- Gordon K, et al. Late-breaking abstract, AAD Virtual Meeting Experience, 12-14 June 2020.
Posted on
Previous Article
« Good response and pruritus reduction in AD with novel selective JAK1 inhibitor Next Article
A new topical PDE-4 inhibitor effective in psoriasis »
« Good response and pruritus reduction in AD with novel selective JAK1 inhibitor Next Article
A new topical PDE-4 inhibitor effective in psoriasis »
Table of Contents: AAD 2020
Featured articles
Late-Breaking Abstracts
IL-17A and IL-17F blockade remarkably effective in psoriasis
Good response and pruritus reduction in AD with novel selective JAK1 inhibitor
Novel IL-23 blocker risankizumab highly effective and tolerable in psoriasis
Tape stripping – a painless way to distinguish AD and psoriasis?
IL-4/IL-13 blocker dupilumab effective in children with severe AD
Pembrolizumab leads to higher toxicity risk in obese melanoma patients
Can gene expression help to pick the right biologic to treat psoriasis in cancer patients?
Omalizumab for cancer-induced dermatoses
Psoriasis – What Is Hot?
Psoriasis therapy for children and pregnancies
Biologic psoriasis treatment to lower cardiovascular risk?
Systemic Therapies for Dermatologists
How to manage cutaneous side effects of immunotherapy
Cannabinoids: a future role in dermatology?
Hidradenitis Suppurativa/Acne Inversa
Biologics in HS – a growing armamentarium
Pearls of the Posters
Selective IL-23 blocker safe in elderly psoriasis patients
Spironolactone safe for androgenetic alopecia in cancer survivors
Baricitinib beneficial in head and neck AD
ECLIPSE trial: skin clearance independent of PsA status at baseline
Related Articles
August 6, 2020
IL-17A and IL-17F blockade remarkably effective in psoriasis
August 19, 2020
Continuous terbinafine most effective for toenail onychomycosis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

