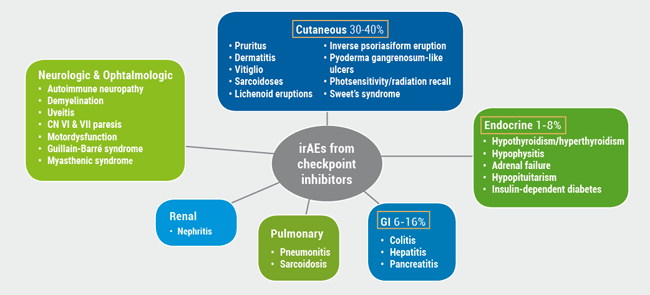
Immune checkpoint inhibitors like the anti-programmed cell death receptor (PD)-1 antibodies and PD-ligand 1 (L1) receptors have emerged as a frontline treatment for a growing list of malignancies. Unfortunately, their use can be limited by multiple irAEs (see Figure) [2]. Immune checkpoint inhibitors can cause a spectrum of AEs, essentially any organ can be involved, most commonly on the skin: 30-40% of patients will have some cutaneous eruptions, Prof. Jennifer N. Choi (Northwestern Feinberg School of Medicine, USA) explained [2].
Figure: Cutaneous side effects of immunotherapy [Adapted from 1]
Patients with dermal side effects respond better to anti-cancer therapy
Cutaneous toxicity is a predictive biomarker for clinical outcome in patients receiving the 3 main classes of anti-cancer therapy: molecularly targeted kinase inhibitors, immune checkpoint inhibitors, and cytotoxic chemotherapeutics [3]. A positive association has been observed between dermatitis after PD-1/PD-L1 inhibitor therapy and progression-free survival and overall survival [4].
Rash is the most frequent AE reported with anti-PD-1 therapies, with a median time to onset of 4-6 weeks. Eczema and lichenoid dermatitis are also common in these patients. The clinical pattern of lichenoid dermatitis can be extremely heterogeneous and can be either localised, generalised, palmoplantar, or mucosal [5]. This also holds true for the time to onset, which can be 3 days to 13 months. In a study including 20 patients developing lichenoid eruptions due to PD-1 or PD-L1 antibodies, 80% of patients reported concurrent medications previously reported to cause lichenoid drug eruptions [5]. In this study, 80% of cases had a clinical morphology consisting of erythematous papules with scale in a variety of distributions. These drug eruptions were generally manageable with topical steroids.
In case of combination therapy with nivolumab and ipilimumab, dermal toxicity is enhanced [6]. The National Comprehensive Cancer Network published a guideline regarding the management of immunotherapy-related toxicity [7]. In this guideline, recommendations for continuing or halting immunotherapy can be found, depending on the severity of the toxicity. For management of maculopapular rash, topical steroids are the first choice [7].
Life-threatening side effects are possible
Rarely, anti-PD-1 cutaneous eruptions can be life threatening: immunotherapy can cause toxic epidermal necrolysis (TEN) and Steven-Johnsons Syndrome (SMS). A TEN-like reaction was associated with nivolumab therapy following ipilimumab [8]. In these cases, immunotherapy has to be discontinued permanently, and in-patient care is required [7].
Approximately 30% of patients suffer from immunotherapy-induced pruritus, which is associated with rash and xerosis. The underlying cause is an enhanced immune system activation in the skin. High potency topical steroids are the first choice in this case. In patients with moderate pruritus, topical steroids should be combined with oral antihistamines [7].
Vitiligo in up to a quarter of patients
Vitiligo is another dermal toxicity, which occurs in 8-25% of patients with a variable time to onset from 52-453 days [5]. In a retrospective study, melanoma patients developing vitiligo following therapy with pembrolizumab had a significantly longer progression-free survival compared with patients who did not develop cutaneous AEs [9]. Published case reports show that repigmentation of vitiligo was associated with melanoma relapse [10].
Other anti-PD-1 side effects are psoriasiform eruptions or an exacerbation of psoriasis [5,11]. A history of psoriasis was present in 71% of cases. Mean time of onset between anti-PD-1 initiation and psoriasis flare is 50 days. “Patients with a known history of psoriasis should carefully be monitored during immunotherapy,” Prof. Choi recommended. Topical steroid treatment should be initiated early on.
Other dermatologic toxicities that have been published are autoimmune bullous skin conditions, for example bullous pemphigoid [12]. Treatment of choice in mild cases are topical steroids. In severe cases, rituximab has shown to be effective [7].
- Choi JN. AAD Virtual Meeting Experience, 12-14 June 2020.
- Hofmann L, et al. Eur J Cancer 2016;60:190-209.
- Rzepecki AK, et al. J Am Acad Dermatol 2018;79:545-555.
- Lee CKM, et al. J Am Acad Dermatol 2018;79:1047-1052.
- Shi VJ, et al. JAMA Dermatology 2016;152:1128-1136.
- Wolchok JD, et al. N Engl J Med 2013:369:122-133.
- Thompson JA, et al. J Natl Compr Canc Netw 2019:17:255-289.
- Nayar N, et al. J Immunother 2016;39:149-152.
- Sanlorenzo M, et al. JAMA Dermatol 2015;151:1206-1212.
- Nakamura Y, et al. JAMA Dermatol 2017;153:942-944.
- Voudouri D, et al. Curr Probl Cancer 2017;41:407-412.
- Naidoo J, et al. Cancer Immunol Res 2016;4:383-389.
Posted on
Previous Article
« Cannabinoids: a future role in dermatology? Next Article
Biologics in HS – a growing armamentarium »
« Cannabinoids: a future role in dermatology? Next Article
Biologics in HS – a growing armamentarium »
Table of Contents: AAD 2020
Featured articles
Late-Breaking Abstracts
IL-17A and IL-17F blockade remarkably effective in psoriasis
Good response and pruritus reduction in AD with novel selective JAK1 inhibitor
Novel IL-23 blocker risankizumab highly effective and tolerable in psoriasis
Tape stripping – a painless way to distinguish AD and psoriasis?
IL-4/IL-13 blocker dupilumab effective in children with severe AD
Pembrolizumab leads to higher toxicity risk in obese melanoma patients
Can gene expression help to pick the right biologic to treat psoriasis in cancer patients?
Omalizumab for cancer-induced dermatoses
Psoriasis – What Is Hot?
Psoriasis therapy for children and pregnancies
Biologic psoriasis treatment to lower cardiovascular risk?
Systemic Therapies for Dermatologists
How to manage cutaneous side effects of immunotherapy
Cannabinoids: a future role in dermatology?
Hidradenitis Suppurativa/Acne Inversa
Biologics in HS – a growing armamentarium
Pearls of the Posters
Selective IL-23 blocker safe in elderly psoriasis patients
Spironolactone safe for androgenetic alopecia in cancer survivors
Baricitinib beneficial in head and neck AD
ECLIPSE trial: skin clearance independent of PsA status at baseline
Related Articles
August 6, 2020
Omalizumab for cancer-induced dermatoses
August 6, 2020
Biologic psoriasis treatment to lower cardiovascular risk?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

