The oral JAK1/JAK2 inhibitor baricitinib has already proven its efficacy versus placebo in treating moderate-to-severe AD in the identical, double-blind, randomised, controlled, phase 3 BREEZE-AD1 and BREEZE-AD2 trials. Patients with AD often experience affection of head and neck as particularly inconvenient, resulting in a negative impact on their quality of life. Yet, AD in these locations is difficult to treat.
Thus, pooled data from over 1,200 patients in BREEZE-AD1 and BREEZE-AD2 was used to assess efficacy of baricitinib on head and neck AD. The trial included adult patients with a history of moderate-to-severe AD for ≥12 months. They all had inadequate response to at least 1 topical treatment within the last 6 months or had shown intolerance to this therapy. Depending on earlier use of topical or systemic treatment, there was a washout period of 2-4 weeks prior to the trial. Topical steroids were only allowed as rescue therapy. Median age of the participants was 33 years, 38% were female, and 98.1% of the study participants had head and/or neck involvement.
Patients were randomised to 4 groups 2:1:1:1 receiving either placebo, or baricitinib at doses of 1 mg (BARI 1 mg), 2 mg (BARI 2 mg), or 4 mg (BARI 4 mg) per day over 16 weeks. Percentage change in Eczema Area and Severity Index (EASI) (in the intention-to-treat population) and EASI 50 and EASI 75 responders were assessed by mixed model repeated measure and logistic regression (see Figure). At baseline, the mean EASI subscore for head/neck was 31.7, head/neck EASI for erythema 2.3.
Figure: EASI 50/75 response rates in the intention-to-treat population [1]
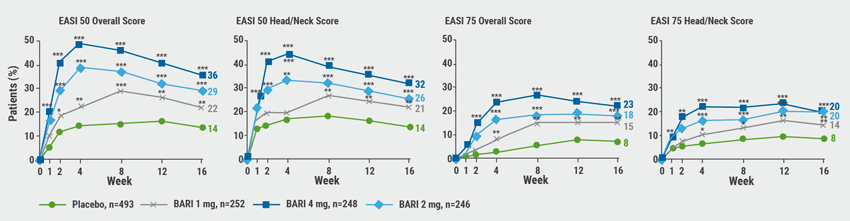
BARI, baricitinib; EASI, Eczema Area and Severity Index; PBO, placebo.
*P<0.05, **P<0.01, ***P<0.001 versus placebo.
The investigators found significant amelioration rates in the head/neck area compared with placebo already after 1 week with 5.3% (placebo), 18.2% (BARI 2 mg), and 23.0% (BARI 4 mg). Corresponding improvement for the overall EASI were 8.9%, 24.1%, and 28.6% (P<0.001 for each BARI group vs placebo). At week 16, an improvement of the EASI by 50% (EASI 50) for head/neck was reached by 25.6% and 31.9% In the BARI 2 mg and BARI 4 mg groups versus 13.8% in the placebo group (P<0.001 for both dosages of baricitinib). An EASI 75 head/neck erythema response could be observed as early as week 2 in the BARI 2 mg and BARI 4 mg groups with significant test results compared with placebo (P≤0.05). There was no new or unexpected safety profile. All in all, baricitinib was effective in reducing the severity of AD involvement in the head and neck region.
For more articles on Type 2 Inflammation, see our independent webportal inflammation-type2.org.
- Simpson EL, et al. P15059, AAD Virtual Meeting Experience, 12-14 June 2020.
Posted on
Previous Article
« Continuous terbinafine most effective for toenail onychomycosis Next Article
Spironolactone safe for androgenetic alopecia in cancer survivors »
« Continuous terbinafine most effective for toenail onychomycosis Next Article
Spironolactone safe for androgenetic alopecia in cancer survivors »
Table of Contents: AAD 2020
Featured articles
Late-Breaking Abstracts
IL-17A and IL-17F blockade remarkably effective in psoriasis
Good response and pruritus reduction in AD with novel selective JAK1 inhibitor
Novel IL-23 blocker risankizumab highly effective and tolerable in psoriasis
Tape stripping – a painless way to distinguish AD and psoriasis?
IL-4/IL-13 blocker dupilumab effective in children with severe AD
Pembrolizumab leads to higher toxicity risk in obese melanoma patients
Can gene expression help to pick the right biologic to treat psoriasis in cancer patients?
Omalizumab for cancer-induced dermatoses
Psoriasis – What Is Hot?
Psoriasis therapy for children and pregnancies
Biologic psoriasis treatment to lower cardiovascular risk?
Systemic Therapies for Dermatologists
How to manage cutaneous side effects of immunotherapy
Cannabinoids: a future role in dermatology?
Hidradenitis Suppurativa/Acne Inversa
Biologics in HS – a growing armamentarium
Pearls of the Posters
Selective IL-23 blocker safe in elderly psoriasis patients
Spironolactone safe for androgenetic alopecia in cancer survivors
Baricitinib beneficial in head and neck AD
ECLIPSE trial: skin clearance independent of PsA status at baseline
Related Articles
August 6, 2020
Biologics in HS – a growing armamentarium
August 6, 2020
Biologic psoriasis treatment to lower cardiovascular risk?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

