Apart from highly potent corticosteroids, the main topical treatment options in psoriasis consist of vitamin D analogues and retinoids. These drugs are not always well tolerated due to their side effects, especially over longer periods of time. “Specifically, we are worried about using corticosteroids or non- corticosteroids in facial and intertriginous areas because of irritation and other side effects like the formation of stretch marks,” stressed Prof. Mark Lebwohl (Icahn School of Medicine at Mount Sinai, USA). The new topical treatment roflumilast inhibits phosphodiesterase (PDE)-4, which is known to be elevated in psoriatic skin lesions, thus influencing different cytokines like TNFα, IFNγ, IL-17, and IL-23.
The current phase 2b study was designed as a double-blind, randomised, parallel-group, vehicle-controlled trial. Included were 331 adult patients with chronic plaque psoriasis and an Investigator’s Global Assessment (IGA) ≥2. Patients were randomised to receive once daily treatment with roflumilast cream 0.3%, 0.15%, or a vehicle over 12 weeks. The primary endpoint was an IGA score of 0 or 1 (clear or almost clear) at week 6. Among other parameters, intertriginous IGA success rates were also evaluated. Baseline characteristics like age, sex, and disease severity were comparable between the groups. The exception was a higher proportion of patients with lesions of a specially created intertriginous IGA (I-IGA) score 3 (i.e. moderate) in the 0.3% group (50%) than in the 0.15% group (16.7%), but this was comparable to the vehicle group (47.1%).
Both doses of roflumilast reached the primary endpoint. They showed a significant and dose-dependent superiority versus vehicle (roflumilast 0.3% P<0.001; roflumilast 0.15% P=0.004). The peak improvement did not occur at week 6 but at week 8 (see Figure). The effect was rapid, with both concentrations being significantly more effective than vehicle as early as week 2. IGA achievement of 0/1 plus 2 grade improvement was reached by 32.2% (roflumilast 0.3%) and 24.5% (roflumilast 0.15%) of the patients at week 8 versus a respective percentage of 9.8% in the vehicle group (P≤0.005). Interestingly, the new agent also showed very good results at 0.3% in the topically difficult-to-treat intertriginous areas with over 90% of clear or almost clear plus 2 grade improvement from baseline at week 12.
Figure: Roflumilast cream led to early improvement in PASI score compared with the vehicle [1]
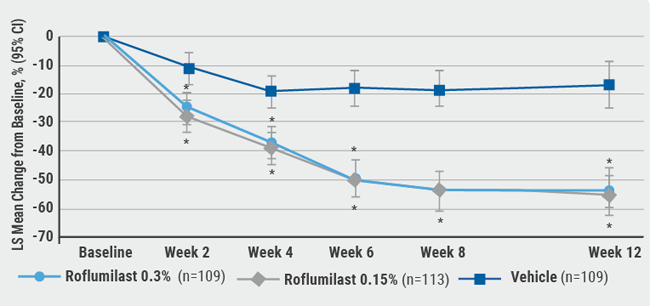
CI, confidence interval; LS, least squares, PASI, Psoriasis Area and Severity Index. Data for intention-to-treat population.
*P<0.001 versus vehicle.
The patient-reported itch, measured by worst itch-Numeric Rating Scale, was significantly reduced by the higher dose of roflumilast (0.3%). The overall burden of disease started to lessen from week 2 and continued to decrease significantly for both dosages of the agent versus vehicle through week 12. “The adverse events seen with this cream were negligible,” added Prof. Lebwohl. Most treatment-emergent adverse events were mild or moderate. Only 1 patient treated with 0.3% roflumilast discontinued treatment due to adverse events, none of those treated with 0.15% roflumilast, and 2 patients from the vehicle group.
- Lebwohl MG, et al. Late-breaking abstract, AAD Virtual Meeting Experience, 12-14 June 2020.
Posted on


