VTE among paediatric patients differs substantially from adults in many aspects, including aetiology, anatomical location, frequency, and recovery. This different natural history of VTE in children, coupled with metabolic variation based on age and body weight, complicate the anticoagulant options available in daily practice. No formulations of direct oral anticoagulants —either liquid (for small children) or tablet— are currently available for children with VTE; this unmet need drove the current study.
Prof. Guy Young (Children's Hospital Los Angeles, USA) reported the dose-exposure versus response of rivaroxaban used in EINSTEIN-Jr [1]. Age-adjusted and body weight-adjusted dosing of rivaroxaban was incorporated to achieve a similar exposure as that observed in adults treated for VTE with 20 mg rivaroxaban.
Children were randomised 2:1 to weight-based rivaroxaban doses or standard treatment with heparin or vitamin K antagonists. The main study period was 3 months for the majority of children or 1 month for children under 2 years with clear catheter-related VTE, followed by open-label treatment for up to 12 additional months. A total of 316 children received rivaroxaban in tablet form (n=121) or liquid formulation (n=195). Dosing/scheduling was stratified by body weight: children weighing ≥30 kg received daily dosing, children weighing 12-29 kg received twice-daily dosing, and children weighing <12 kg received 3 doses daily. In order to span the broad 2.6-50 kg range among the children in the trial, 12 separate doses were used. Age was also a factor in the algorithm used: groups were 0-6 months (n=13), 6-23 months (n=21), 2-5 years (n=44), 6-11 years (n=65), and 12-17 years (n=173).
The principal safety outcome was major bleeding and clinically relevant non-major bleeding. No major bleeding events were reported, although 10 patients experienced clinically relevant non-major bleeding and another 111 patients had minor bleeding events. Imaging outcomes at 3 months were classified as healthy/no disease evident (n=124), improved (n=125), no relevant change (n=16), deteriorated (n=1), or unclear (n=48).
Three standard pharmacokinetic measures were used to confirm dosing: area under the 24-hour curve for blood levels (24-hour AUC), peak concentration, and trough concentration. In total, 25 patients had measures out of the target ranges. Only 5 of those exceeded the maximum for any of the 3 measures at any time, so excessive exposure was not significant. No patient had a trough concentration below the target range. Notably, only 1 patient with values outside of target had a suboptimal clinical outcome (24-hour AUC below target, no clinical improvement). There were no differences observed between the tablet and liquid formulations.
In conclusion, weight-adjusted dosing of rivaroxaban in children with VTE was well tolerated and demonstrated anti-coagulant activity.
1. Young G, et al. Abstract 164, ASH 2019, 7-10 December, Orlando, USA.
Posted on
Previous Article
« Arginine supplements help against sickle cell disease pain Next Article
BCMA-targeted CAR T therapy yields 100% response in relapsed/refractory MM »
« Arginine supplements help against sickle cell disease pain Next Article
BCMA-targeted CAR T therapy yields 100% response in relapsed/refractory MM »
Table of Contents: ASH 2019
Featured articles
Late-Breaking Abstracts
Likely new standard of care: Blinatumomab for children with relapsed B-ALL
Pivotal phase 3 trial in cold agglutinin disease: sutimlimab can stop haemolysis
Oral azacitidine improves overall survival in patients with AML in remission
BCL11A as a novel target in gene therapy for sickle cell disease
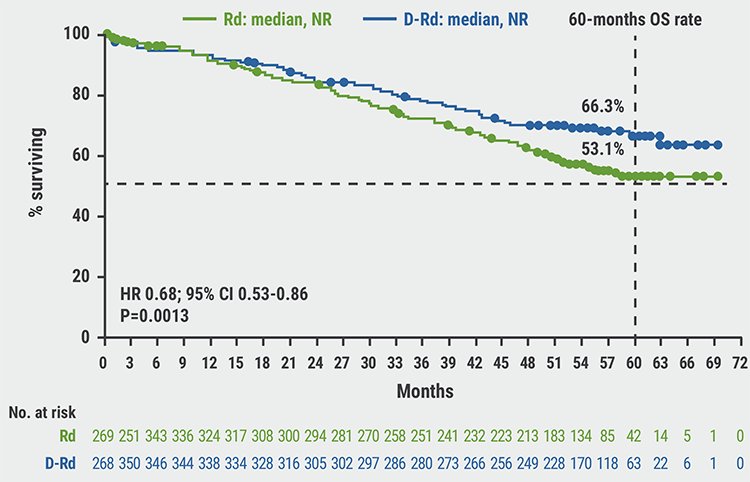
Adding daratumumab to carfilzomib/dexamethasone prolongs PFS and OS in R/R MM
Long-term data of ropeginterferon alpha-2b in polycythaemia vera
Anti-CD70 is safe with hypomethylating agents in AML
MRD assessment to guide pre-emptive treatment decisions
Luspatercept effective for myelofibrosis-associated anaemia
Arsenic, ATRA, and ascorbic acid in acute promyelocytic leukaemia maintenance
Updated results ECOG-ACRIN E2906: decitabine maintenance after alloSCT
Sickle Cell Disease
Arginine supplements help against sickle cell disease pain
Abatacept prevents graft-versus-host disease in sickle cell patients after alloSCT
Plenary Scientific Session
HOVON-96: Better outcomes with cyclophosphamide after transplantation
Erythroferrone and skeletal changes associated with thalassaemia
Experimental model for limitations of haematopoietic stem cells propagation
Mosunetuzumab: complete remissions in non-Hodgkin lymphoma
Inclusive Medicine
Socioeconomic disparities and survival in paediatric AML
Oral selinexor/pomalidomide/dexamethasone shows activity in heavily pre-treated multiple myeloma
CAR T-cell therapy successful in older non-Hodgkin’s lymphoma patients
Mild renal impairment in African Americans does not affect OS in AML
ALCYONE: New overall survival results for myeloma
Venous Thromboembolism
Rivaroxaban is safe and effective for paediatric venous thromboembolism
Aspirin plus DOAC is not better than a DOAC alone
20-Year follow-up of imatinib in chronic myeloid leukaemia after failure with interferon
CAR T and Beyond
BCMA-targeted CAR T therapy yields 100% response in relapsed/refractory MM
Anti-BCMA/anti-CD38 in refractory multiple myeloma
Related Articles
August 9, 2019
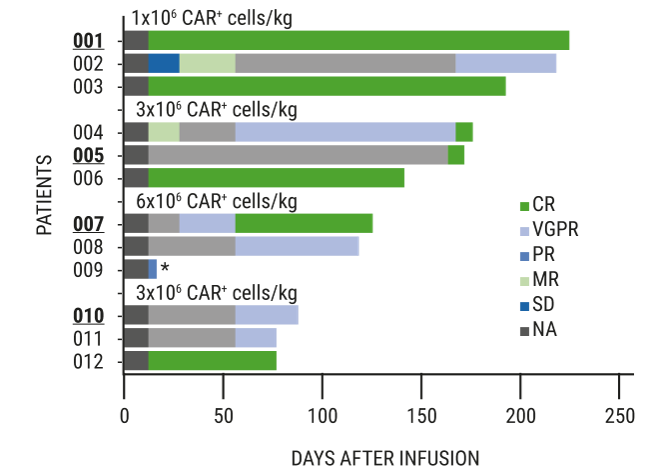
Chimeric antigen receptor T cell therapy in multiple myeloma
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

