Although it is widely acknowledged that age and BMI are important factors in cancer patients who are treated with chemotherapy, the same is not quite clear with regard to treatment with immune checkpoint inhibitors. A pooled analysis of age by Marur et al. in 2018 demonstrated that the age of the patient did not have any impact on the clinical response to checkpoint inhibitors [1]. With regard to BMI, it was shown earlier that obese patients (BMI >35) who suffered from melanoma or renal cell carcinoma had better outcomes [2-4].
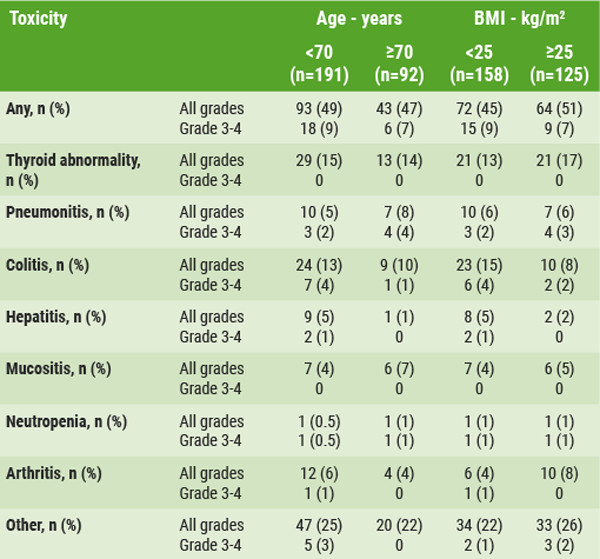
Thus, Corentin et al. aimed to investigate the role of age and BMI in NSCLC patients who were eligible for treatment with immune checkpoint inhibitors. This was done in a retrospective analysis which included 381 advanced NSCLC patients from 3 centres in France and Canada who received treatment with immune checkpoint inhibitors. These 3 cohorts (n=381) were of similar median age (±65 years). Median BMI was around 24.5, with little over half of patients being overweight. The majority of patients had ≥2 lines of treatment. Multivariate analysis using multiple Cox regression showed that both overall survival and progression-free survival were not impacted by either age or BMI. The incidence of all grade irAEs in patients aged <70 years (n=191) and those aged ≥70 (n=92) was 49% and 47%, respectively. When stratified to BMI, all grade irAEs occurred in 46% of patients with BMI <25 (n=158) vs 51% in those with BMI ≥25 (n=125). All observed irAEs are displayed in the Table [5]. Thus, in spite of what has been reported before, age and BMI are not associated with efficacy outcomes in NSCLC patients who are treated with immune checkpoint inhibitors [5].
Table. The incidence of irAEs in NSCLC patients treated with immune checkpoint inhibitors, stratified by age/BMI [5]
- Marur S, et al. Semin Oncol. 2018;45(4):220-225.
- Fang S, et al. J Invest Dermatol. 2017;137(8):1792-1795.
- Albiges L, et al. J Clin Oncol. 2016;34(30):3655-36633.
- Bagheri M, et al. Int J Obes. 2016;40(12):1817-1822.
- Corentin R, et al. P1.04-01. WCLC 2019.
Posted on
Previous Article
« Durvalumab added to etoposide improves outcomes in ES-SCLC Next Article
Use of clinical registries in phase 4 of DMT »
« Durvalumab added to etoposide improves outcomes in ES-SCLC Next Article
Use of clinical registries in phase 4 of DMT »
Table of Contents: WCLC 2019
Featured articles
Five-fold increase of OS at 5 years with nivolumab vs docetaxel
Screening, Detection, and Diagnosis
Non-Small-Cell Lung Cancer
Five-fold increase of OS at 5 years with nivolumab vs docetaxel
Promising phase 1 results of novel KRAS-inhibitor in NSCLC
Selpercatinib (LOXO-292) shows durable activity in RET fusion-positive lung cancer
Other Thoracic Malignancies
Immuno-Oncology
Nivolumab + ipilimumab safe first-line treatment for NSCLC patients with comorbidities
Targeted Therapy
Phase 3 Trial Updates
First-line pembrolizumab monotherapy offers durable OS benefit vs chemotherapy in NSCLC patients with high PD-L1 expression
Related Articles

October 29, 2020
Biomarkers: a novel tool to improve lung nodule classification
June 25, 2019
Electromagnetic navigation bronchoscopy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

