Hospital-acquired bacterial pneumonia is a critical concern in hospitals, with VAP remaining the most common infection in the ICU. VAP infection is often due to Staphylococcus aureus, an increasingly difficult-to-treat pathogen. Respiratory tract colonisation precedes VAP in 94% of the cases; the authors’ rationale for this study was pre-emptive intervention for the patients at higher risk of the disease. Suvratoxumab is an extended half-life human monoclonal antibody that targets the pore-forming alpha toxin produced by Staphylococcus aureus.
The participants in this trial were critically ill patients who were intubated and had been on mechanical ventilation for ≥3 days with the clinical expectation of at least 3 more days of ventilation, without evidence of pneumonia. Patients were placed in intensive care units during the length of the investigation. The participants (n=196) had confirmed Staphylococcus aureus in their lower respiratory tracts (as assayed by polymerase chain reaction [PCR]) with no traces of related diseases. PCR-positive patients were randomised to receive an intravenous infusion of suvratoxumab (n=96) or placebo (n=100) and were assessed for pneumonia for 30 days and monitored completely for 90 days.
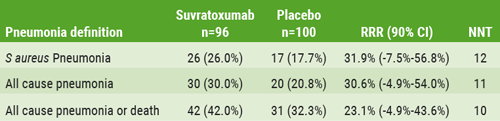
The primary endpoint was the incidence of Staphylococcus aureus pneumonia within 30 days post-dose, as determined by clinical, radiographic, and microbiologic negative assessments, adjudicated by an independent panel of blinded VAP experts and radiologists. In addition, the trial’s secondary outcomes included pharmacokinetic (PK) parameters and anti-drug antibody responses 90 days post-dose. The attack-rate of Staphylococcus aureus VAP in the PCR-positive population was 26% in the placebo group vs 17.7% in the suvratoxumab group. The relative risk reduction was 31.9% (90% CI -7.5% to 56.8%) in favour of pre-emptive suvratoxumab.
In a subgroup analysis, the benefit of the drug was even more evident in patients younger than 65 years, with a 47.4% relative risk reduction (90% CI 3.5% to 71.4%). Analysis of healthcare resource utilisation savings through 90 days post-dose showed significant reduction in days of hospitalisation, days in ICU, and mechanical ventilation, making this a cost-effective approach. Serum PK was sustained above the target for up to 60 days, and the population PK-determined half-life was 72±33 days. In the placebo group, anti-drug antibodies were detected post-dose (<5%) but none (0%) were detected in the suvratoxumab group. No differences were reported in adverse events between the 2 groups. Efficacy was observed even when patients received a full VAP bundle. The 30-day mortality results were balanced; there was no mortality signal. In conclusion, suvratoxumab demonstrated acceptable safety and pharmacokinetic, while offering clinical benefit to a subset of patients.
Table: Efficacy against pneumonia definitions in mITT population [1]
- François B, et al. A7358, ATS 2019, 17-22 May, Dallas, USA.
Posted on
Previous Article
« Treatable traits in chronic inflammatory airway disease: back to basics Next Article
E-cigarette use disrupts normal immune response to viral infections, particularly in women »
« Treatable traits in chronic inflammatory airway disease: back to basics Next Article
E-cigarette use disrupts normal immune response to viral infections, particularly in women »
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

