Nephrectomy is the standard of care for the treatment of locoregional RCC. Nearly half of patients eventually experience disease recurrence after surgery. Patients with M1 stage and no evidence of disease (NED) after resection of oligometastatic sites are also at high risk of relapse. Relapse after surgery for high-risk clear-cell RCC (ccRCC) is associated with shortened survival. To reduce the relapse risk, effective perioperative treatments are needed, and adjuvant immune therapy might offer an attractive potential strategy for these patients.
The phase 3, double-blind, multicentre KEYNOTE-564 trial (NCT03142334) investigates pembrolizumab versus placebo in patients with ccRCC [1]. Patients with histologically confirmed ccRCC (n=994) were randomised 1:1 to pembrolizumab 200 mg or placebo every 3 weeks for ~1 year. Patients were in one of the following risk groups:
- pT2, grade 4 or sarcomatoid, N0, M0;
- pT3 or pT4 any grade, N0, M0;
- pT4 any grade, N0, M0;
- pT any stage, any grade, N+, M0; or
- M1 with NED after surgery of primary tumour and resection of oligometastatic lesions.
Prof. Thomas Powles (Barts Cancer Centre, London, UK) shared the results of the study.
After a median follow-up of 24.1 months, the primary endpoint of disease-free survival (DFS) was met. Median DFS was not reached for both arms. The estimated DFS rate at 24 months was 77.3% with pembrolizumab versus 68.1% with placebo (HR 0.68; 95% CI 0.53–0.87; P=0.0010; see Figure). Overall, DFS benefit was consistent across risk subgroups.
Figure: DFS by investigator in the intention-to-treat population [1]
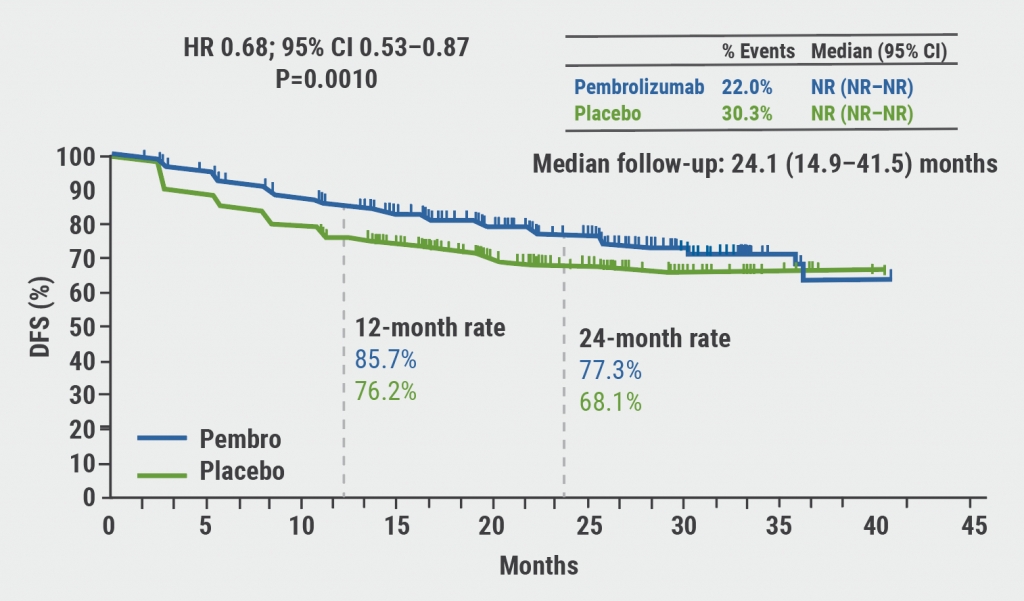
Figure kindly provided by Prof. Powles.
The key secondary endpoint was overall survival (OS). Interim results demonstrated an estimated OS rate at 24 months of 96.6% with pembrolizumab versus 93.5% with placebo. Median OS was not reached for both arms (HR 0.54; 95% CI 0.30–0.96; P=0.0164). Due to a prespecified p-value boundary, these results were not significant.
Safety results were in line with expectations; no new safety signals were observed. Grade 3–5 all-cause adverse events occurred in 32.4% with pembrolizumab and in 17.7% with placebo. No treatment-related deaths occurred with pembrolizumab.
Adjuvant pembrolizumab after nephrectomy demonstrated a statistically significant and clinically meaningful improvement in DFS compared with placebo in patients with intermediate-high, high-risk, or M1 NED ccRCC. The benefit was consistent across subgroups, including the M1 NED population, potentially extending the use of pembrolizumab to these patients. Although the number of participants who had partial nephrectomy was small, DFS benefit was consistent in this population. Additional follow-up is planned for the key secondary endpoint of OS.
- Powles T. Pembrolizumab (pembro) vs. placebo as post nephrectomy adjuvant therapy for patients (pts) with renal cell carcinoma (RCC): randomized, double-blind, phase 3 KEYNOTE-564 study. Game changing session 4. EAU21 Virtual, 8–12 July 2021.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« PSMA PET-CT more accurate than standard-of-care imaging in RCC Next Article
Best of EAU: Immune cell populations have prognostic value in RCC »
« PSMA PET-CT more accurate than standard-of-care imaging in RCC Next Article
Best of EAU: Immune cell populations have prognostic value in RCC »
Table of Contents: EAU 2021
Featured articles
EAU TV: Robotic surgery and advanced prostate cancer
LUTS & BPH
Best of EAU: The surgical armamentarium is evolving
IPSS: Visual alternatives to aid comprehension and new risk prediction models
Urinary Tract Infections
Prophylactic treatments for recurrent urinary tract infections
Failure of conservative management in emphysematous pyelonephritis
Antibiotic treatment of healthcare-associated infections
Prostate Cancer
EAU TV: Robotic surgery and advanced prostate cancer
EAU TV: The best on prostate cancer and incontinence & andrology
Best of EAU: Updates on imaging and treatment of prostate cancer
Radiographic PFS benefit of adding abiraterone to ADT and docetaxel in mCSPC
177Lu-PSMA-617: A new class of effective therapy
Testis and Penile Cancer
Best of EAU: New advances in testicular and penile cancer
Recommendations for the management of indeterminate small testis masses
Residual tumour resection in case of elevated markers
Bladder Cancer
Best of EAU: Highlights on bladder cancer
ctDNA can guide adjuvant immunotherapy in muscle-invasive bladder cancer
Durvalumab ± tremelimumab by cisplatin eligibility in metastatic urothelial carcinoma
Circulating tumour cells could aid in the decision to give neoadjuvant chemotherapy
Renal Cancer
Best of EAU: Immune cell populations have prognostic value in RCC
KEYNOTE-564: First positive phase 3 results with adjuvant checkpoint inhibition in RCC
PSMA PET-CT more accurate than standard-of-care imaging in RCC
Worse renal function after radical versus partial nephrectomy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy


