“ctDNA has substantial potential to be used throughout the individual patient journey,” Prof. Jürgen Gschwend (Technical University Munich, Germany) explained. It could be useful in many phases, from screening to monitoring for resistance in post-treatment care. For example, ctDNA could be useful to determine the need for treatment intensification, such as the need for perioperative therapy. Post-surgery presence of ctDNA in patients with bladder cancer indicates molecular disease progression that precedes clinical relapse. Prof. Gschwend argued that the use of this marker may shorten drug developmental timelines and may help to avoid treating patients who do not require additional therapy.
The global, phase 3 IMvigor010 study (NCT02450331) compared adjuvant atezolizumab with observation in patients with MIBC following surgery. Of the 809 patients included, 581 (72%) had biomarker-evaluable tissue.
The primary endpoint of disease-free survival (DFS) was not met: DFS was similar between the intention-to-treat (ITT) and the biomarker-evaluable patient cohort. The same was true for overall survival (OS). “However, an important finding was that ctDNA-positive patients had a significantly improved DFS and OS with atezolizumab compared with observation,” added Prof. Gschwend.
Figure. DFS and OS with atezolizumab compared with observation in ctDNA-positive patients.
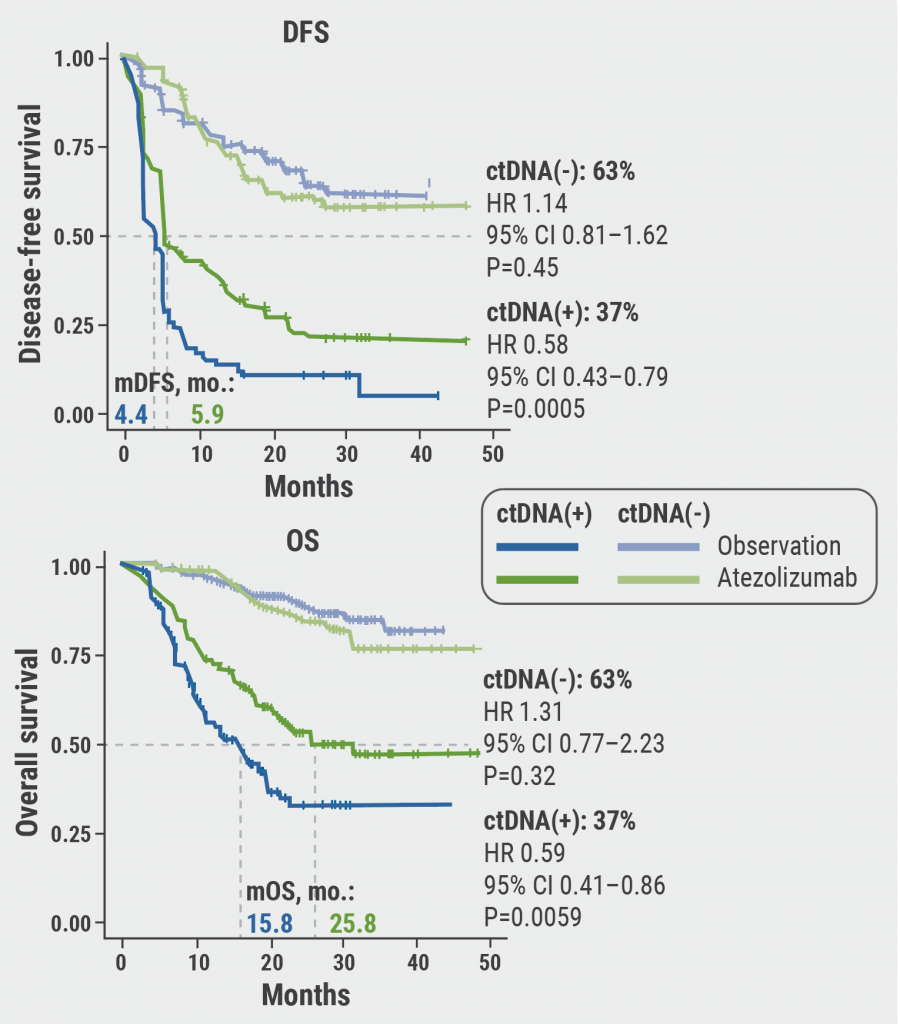
DFS, disease-free survival; OS, overall survival.
Figure kindly provided by Prof. Jürgen Gschwend.
Moreover, results from IMvigor010 confirmed the prognostic value of ctDNA status. ctDNA-positive patients had worse DFS and OS rates compared with ctDNA-negative patients. Furthermore, ctDNA-positive patients who received adjuvant atezolizumab performed better than patients who were under observation.
The combination of ctDNA and high tumour mutation burden translated into a more pronounced DFS and OS benefit compared with tumour mutation burden-negative patients. In addition, ctDNA-positive patients treated with atezolizumab were more likely to clear ctDNA (18% vs 3%), and clearance was associated with improved DFS. OS results were similar.
The IMvigor010 study warrants validation in the currently active IMvigor011 study (NCT04660344). IMvigor011 is recruiting patients with high-risk MIBC who show ctDNA positivity within 20 months after radical cystectomy. ctDNA-positive patients will receive either atezolizumab or placebo for 1 year. If confirmed, the results may change our understanding of post-surgical care.
- Gschwend JE, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Game changing session 3, EAU21 Virtual, 8–12 July 2021.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Durvalumab ± tremelimumab by cisplatin eligibility in metastatic urothelial carcinoma Next Article
Best of EAU: Highlights on bladder cancer »
« Durvalumab ± tremelimumab by cisplatin eligibility in metastatic urothelial carcinoma Next Article
Best of EAU: Highlights on bladder cancer »
Table of Contents: EAU 2021
Featured articles
EAU TV: Robotic surgery and advanced prostate cancer
LUTS & BPH
Best of EAU: The surgical armamentarium is evolving
IPSS: Visual alternatives to aid comprehension and new risk prediction models
Urinary Tract Infections
Prophylactic treatments for recurrent urinary tract infections
Failure of conservative management in emphysematous pyelonephritis
Antibiotic treatment of healthcare-associated infections
Prostate Cancer
EAU TV: Robotic surgery and advanced prostate cancer
EAU TV: The best on prostate cancer and incontinence & andrology
Best of EAU: Updates on imaging and treatment of prostate cancer
Radiographic PFS benefit of adding abiraterone to ADT and docetaxel in mCSPC
177Lu-PSMA-617: A new class of effective therapy
Testis and Penile Cancer
Best of EAU: New advances in testicular and penile cancer
Recommendations for the management of indeterminate small testis masses
Residual tumour resection in case of elevated markers
Bladder Cancer
Best of EAU: Highlights on bladder cancer
ctDNA can guide adjuvant immunotherapy in muscle-invasive bladder cancer
Durvalumab ± tremelimumab by cisplatin eligibility in metastatic urothelial carcinoma
Circulating tumour cells could aid in the decision to give neoadjuvant chemotherapy
Renal Cancer
Best of EAU: Immune cell populations have prognostic value in RCC
KEYNOTE-564: First positive phase 3 results with adjuvant checkpoint inhibition in RCC
PSMA PET-CT more accurate than standard-of-care imaging in RCC
Worse renal function after radical versus partial nephrectomy
Related Articles
February 13, 2021
Details released for CLEAR study on advanced renal cell carcinoma
August 20, 2020
Debate: upfront cytoreductive nephrectomy or not?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

