Prof. Philip Mease (Swedish Medical Center, WA, USA) discussed several new drugs on the block, with a focus on drugs that have recently been approved or will be approved in the near future, including IL-17 blockers, IL-23 blockers, and JAK inhibitors [2,3]. Results from the phase 2b BE ACTIVE study (NCT02969525) on IL-17A/F blocker bimekizumab and the phase 3 PATERA study (NCT03598751) on IL-17A blocker netakimab are promising [4,5]. “We see good musculoskeletal and skin responses with these medications,” Prof. Mease mentioned, “with a safety profile similar to other IL-17 inhibitors.” Another monoclonal antibody in development is izokibep (ABY-035), a unique bispecific, small molecule potently binding IL-17A and albumin.
IL-23 blockers that are approved for psoriasis and in the pipeline for psoriatic arthritis include risankizumab and tildrakizumab. The IL-23 blocker guselkumab has been approved for both psoriasis and psoriatic arthritis. Unlike the rapid effect observed with some biologics, these drugs may take up to 24 weeks to get a full response. The JAK1 inhibitor upadacitinib and the oral, selective TYK2 inhibitor deucravacitinib have shown promise as well [6,7]. “These new drugs show efficacy and are relatively safe for use in this patient group. Taken together, all treatments we are now able to work with for PsA have the capability of getting a patient in low disease activity or remission, which is a treatment target for us,” Prof. Mease concluded.
Heterogeneous pathogenesis and treatment
“A frequently asked question is how to use all these new agents to optimise care for our patients. That is part of the rationale for the creation of treatment recommendations and guidelines,” Prof. Arthur Kavanaugh (University of California, USA) explained. Management of PsA can be challenging due to its heterogeneity. “We need to choose the treatment that offers the best improvement to the patient across all the affected domains (see Figure).”
Figure: Putative immunopathogenesis of PsA [8]
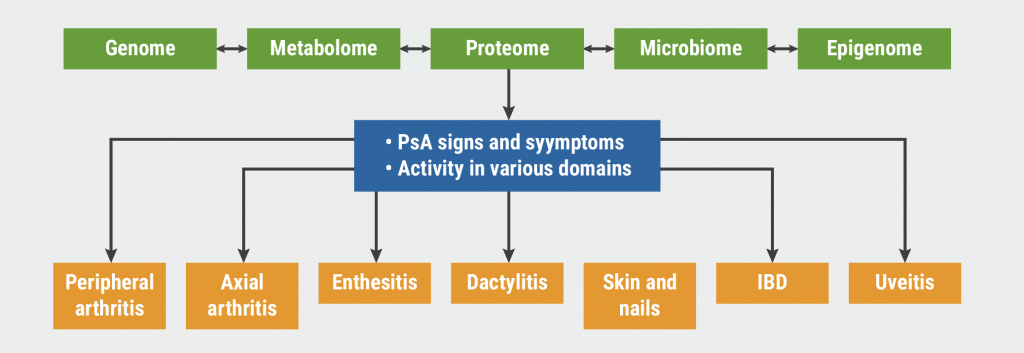
Guidelines since 2009
In 2009, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) published treatment recommendations [9]. The disease was divided into different domains. “Various therapies will work to different extents for different domains specifically. Relatively few choices were available back then, but I think the overall schema is still reasonable,” explained Prof. Kavanaugh.
In the last years, these and other guidelines were regularly updated. In 2018, the guidelines of the American College of Rheumatology (ACR) and the National Psoriasis Foundation (NPF) for the treatment of PsA [11]. One year later, the most recent joint guidelines of the American Academy of Dermatology (AAD) and the NPF was published [12]. In 2019, the EULAR developed recommendations for the treatment of PsA [6].
“The 3rd edition of GRAPPA’s treatment recommendations are a work in progress” [14]. In this version, comorbidities have been broken down into comorbidities and associated conditions. These are important for the choice of treatment for an individual patient.
Interpretations, unmet needs, and future directions
Prof. Kurt de Vlam (UZ Leuven, Belgium) emphasised that these new drugs are a “tremendous improvement for our patients,” but added that not all treatment classes are effective across all key domains of PsA [15].
Prof. Kilian Eyerich (Karolinska Institute, Sweden) and Dr Laura Coates (University of Oxford, UK) discussed unmet needs in the treatment of PsA from a dermatology and rheumatology point of view, respectively. These include the lack of predictive tools, the relatively better treatment outcomes on skin lesions compared with joint problems of PsA, and the need for personalised medicine in PsA. “We still do not know definitively which drug should be given to which patient,” Dr Coates acknowledged.
In the future, Prof. Kavanaugh expects that there will be drugs with new mechanisms of action, combination therapy with different DMARDs and biological DMARDs, and targeted therapy based on cellular and clinical phenotypes. “It is an exciting time for the treatment of PsA, for clinicians and hopefully also for patients.”
- Kavanaugh A. Treatment guidelines/best practice PsA. 6th World Psoriasis & Psoriatic Arthritis Conference, 30 June–3 July 2021.
- Mease P. New treatments/pipeline PsA. 6th World Psoriasis & Psoriatic Arthritis Conference, 30 June–3 July 2021.
- Mease PJ. Rheum Dis Clin North Am. 2015;41:723–38.
- Ritchlin CT, et al. L17, ACR 2018, 19—24 October, Chicago, USA.
- Korotaeva T, et al. OP0226, EULAR 2020, 3–6 June.
- McInnes I, et al. LB0001, EULAR 2020, 3–6 June.
- Armstrong A, et al. POS1042, EULAR 2021, 2–5 June.
- Bravo A, Kavanaugh A. Nat Rev Rheumatol. 2019;15:645–56.
- Ritchlin CT, et al. Ann Rheum Dis. 2009;68:1387–94.
- Coates LC, et al. Arthritis Rheumatol. 2016;68:1060–71.
- Singh JA, et al. Arthritis Rheumatol. 2019;71:5–32.
- Elmets CA, et al. J Am Acad Dermatol. 2019;80:1073–113.
- Gossec L, et al. Ann Rheum Dis. 2020;79:700–12.
- Coates LC, et al. J Rheumatol. 2021:jrheum.201681.
- De Vlam K, Eyerich K, Coates L. Symposium: 20 years of biologics. 6th World Psoriasis & Psoriatic Arthritis Conference, 30 June–3 July 2021.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Patients with PsA have a higher cardiovascular risk Next Article
Pan-European guidelines for the treatment of psoriasis and comorbid conditions »
« Patients with PsA have a higher cardiovascular risk Next Article
Pan-European guidelines for the treatment of psoriasis and comorbid conditions »
Related Articles

September 28, 2021
Could steroid treatment speed recovery from infliximab-induced liver injury?
September 17, 2021
Axial involvement is critical in psoriatic arthritis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

