Evidence on the efficacy and safety of mRNA vaccines in patients with AIIRDs is limited. In a prospective, observational, open-label, controlled multicentre study –presented by Dr Victoria Furer (Tel Aviv Sourasky Medical Center, Israel)– immunogenicity, safety, and efficacy of the Pfizer BioNTech vaccine were investigated in an adult AIIRD population (n=686, mean age 59 years) 2–6 weeks after the second vaccine dose was administered [1]. The vast majority (95.2%) were treated with immunosuppressants. The control group consisted of 121 healthy individuals 2–6 weeks after the second vaccine dose. A value of >15 binding antibody units (BAUs) was considered as a cut-off for seropositivity.
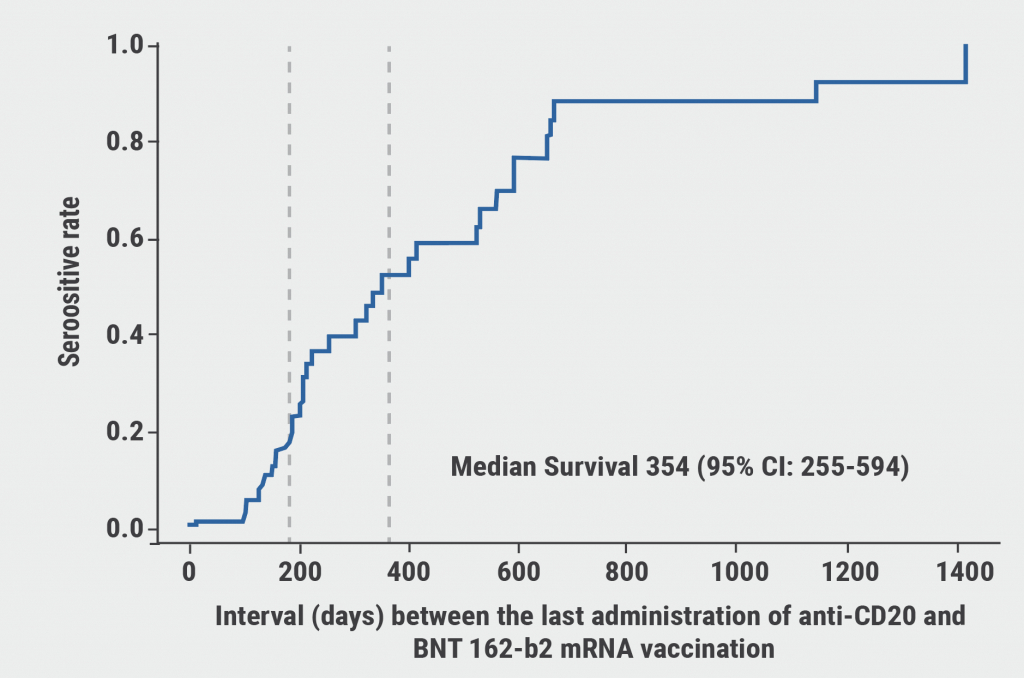
The seropositivity rate was 86% in the AIIRD group versus 100% in the control group. Patients treated with rituximab had the lowest seropositivity rates: 41.4% (OR 0.131; 95% CI 0.07–0.242; P<0.0001). The period between the most recent pre-vaccination administration of rituximab and time of vaccination had a significant effect on the immunogenicity of the vaccine. Seropositivity rate depended on the time of vaccination after the rituximab treatment and was 1.15% at 90 days, 18.39% at 180 days, and 52.18% at 365 days (see Figure).
Figure: The interval between the last administration of rituximab and time of vaccination is associated with seropositivity rate [1]
Other DMARDs that were associated with reduced seropositivity rates were glucocorticoids (66.2%), abatacept (62.5%), mycophenolate mofetil (64.3%), and to a lesser extent methotrexate (84.1%) (see Table). In addition, lower seropositivity rates were more often observed in patients >65 years (79.3%; OR 0.429; P=0.0027) and for certain AIIRD diagnoses: rheumatoid arthritis 82.1% (OR 0.305; P=0.01), idiopathic inflammatory myopathies 36.8% (OR 0.063; P=0.0002), and ANCA-associated vasculitis 30.8% (OR 0.043; P<0.0001).
Table: Seropositivity rates for different DMARDs [1]

Disease activity remained stable after vaccination for most AIIRD patients. One AIIRD patient died 2 weeks after vaccination, and 1 control subject had a mild SARS-CoV-2 infection and fully recovered. Mild AEs after vaccination were comparable in all patient groups. Some cases of herpes zoster (n=6), uveitis (n=2), herpes labialis (n=1), and pericarditis (n=1) were reported in the AIIRD population. Dr Furer argued that, to improve the immunogenicity of the Pfizer BioNTech vaccine, postponing treatment with rituximab or holding treatment with mycophenolate mofetil or abatacept should be considered.
A related study, presented by Dr Pedro Machado (University College London, UK) analysed safety data of COVID-19 vaccines (Pfizer BioNTech 78%, Moderna 5%, AstraZeneca 16%, other 1%) used in RMD patients (n=1,519; mean age 63; 68% women) from the COVAX registry [2]. The diagnostic groups consisted of inflammatory joint diseases (51%), connective tissue diseases (19%), vasculitis (16%), other immune-mediated inflammatory diseases (4%), and non-inflammatory RMDs (9%). All patients had received at least 1 dose of vaccine; 66% were fully vaccinated.
The results demonstrated that the safety profile of the vaccines among patients with RMD was similar to that of the general population. Following vaccination, disease flares were reported in 5% of the patients with inflammatory RMDs. AEs occurred in 31% of the patients. Pain at the injection site (19%), fatigue (11%), headache (7%), and generalised muscle pain (6%) were the most common AEs. Systemic or organ side effects were reported in 33 cases. These AEs were diverse and mostly mild or moderate. Severe AEs were reported in 2 cases: 1 case of transient hemiparesis in a patient with systemic sclerosis and systemic lupus erythematosus, and a case of giant cell arteritis in an elderly patient with osteoarthritis. Dr Machado concluded that the safety profiles of COVID-19 vaccines for RMD patients were reassuring.
- Furer V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD) compared to the general population: a multicenter study. LB0003, EULAR 2021 Virtual Congress, 2–5 June.
- Machado PM, et al. COVID-19 vaccine safety in patients with rheumatic and musculoskeletal disease. LB0002, EULAR 2021 Virtual Congress, 2–5 June.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Passive smoking associated with an increased risk of RA Next Article
Remote management of RA is a feasible alternative for outpatient follow-up »
« Passive smoking associated with an increased risk of RA Next Article
Remote management of RA is a feasible alternative for outpatient follow-up »
Table of Contents: EULAR 2021
Featured articles
COVID-19 Update
Rituximab or JAK inhibitors increase the risk of severe COVID-19
Updates on COVID-19 vaccines in patients with rheumatic disease
Immunomodulatory therapies for severe COVID-19: literature update
New Developments in Rheumatoid Arthritis
JAK inhibitors and bDMARDs not associated with increased risk of serious infections in RA
Remote management of RA is a feasible alternative for outpatient follow-up
TOVERA: Ultrasound is a promising biomarker of early treatment response
The risks of polypharmacy in RA
ABBV-3373: A potential new therapeutic agent for RA
JAK inhibitors and bDMARDs show comparable effectiveness
Spondyloarthritis: Progression in Therapies
SELECT-AXIS: 64-week results of upadacitinib in active ankylosing spondylitis
Guselkumab efficacious in PsA patients with inadequate response to TNF inhibition
Faecal microbiota transplantation not effective in active peripheral PsA
Risankizumab meets primary and ranked secondary endpoints in PsA
Prognostic factors for minimal disease activity in early psoriatic arthritis revealed
Imaging in Large-Vessel Vasculitis
PET/CT is a reliable measure of disease activity in LVV, but does not predict future relapses
Ultrasound is useful for disease monitoring in giant cell arteritis
Prevention in Rheumatic Diseases
Air pollution predicts decreased response to biological treatment in rheumatic diseases
Passive smoking associated with an increased risk of RA
Gene-Environment Interaction in Gout
Gene-diet and gene-weight interactions associated with the risk of gout
What Is New in Systemic Lupus Erythematosus
Intensified treatment regimen of anifrolumab for lupus nephritis is promising
Systemic lupus erythematosus: increased risk of severe infection
Juvenile Idiopathic Arthritis and Osteoarthritis
Efficacy and safety of secukinumab in juvenile idiopathic arthritis
Emerging therapies and future treatment directions in osteoarthritis
Related Articles
December 1, 2023
Repeat steroid injection in knee osteoarthritis possibly beneficial
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

