In the effective management of gout, keeping the level of serum uric acid below its limit of solubility is key, but a substantial part of patients does not reach this therapeutic goal [1,2]. “Tigulixostat is a novel selective xanthine oxidase inhibitor and, unlike allopurinol, it does not have a purine-like backbone structure,” Prof. Robert Terkeltaub (University of California San Diego, CA, USA) described the new agent.
A phase 2 study (NCT03934099) investigated tigulixostat for safety and efficacy in patients with gout and hyperuricemia [1]. The study population consisted of 156 patients with gout, who were randomised to receive either placebo or tigulixostat at dosages of 50 mg, 100 mg, or 200 mg daily over 12 weeks. After that, an additional 2-week safety follow-up was performed. Participants had to have a serum urate level of >6.0 mg/dL if on prior urate-lowering treatment and a value between 8.0 mg/dL and 12 mg/dL after a washout period, or if previously untreated. Colchicine 0.6 mg was given as gout flare prophylaxis. “The primary endpoint was the proportion of subjects achieving serum urates less than 5.0 mg/dL at week 12, as this target is recommended for severe disease including tophaceous gout,” stated Prof. Terkeltaub. The baseline characteristics of the study population included a mean age between 52.0 and 56.6 years, predominantly men, and a mean BMI little above 30 kg/m2. Prof. Terkeltaub also pointed out that about 20% had palpable tophi, and most pre-dosing serum urate levels were <9.8 mg/dL.
At week 12, 47.1% (50 mg), 44.7% (100 mg), and 62.2% (200 mg) of those on the study drug achieved a serum urate <5.0 mg/dL compared with 2.9% on placebo (P<0.0001 for all dosages; see Figure). The rate of patients reaching the secondary endpoint of serum urate <6.0 mg/dL was also significantly higher when on the study drug, with corresponding proportions of 58.8%, 63.2%, and 78.4% versus 2.9%, respectively. In a small active control group of 13 patients receiving febuxostat, 23% reached serum urate values <5.0 mg/dL.
Figure: Rates of study participants who reached the primary endpoint of a serum uric acid level (sUA) <5 mg/dL at week 12 [1]
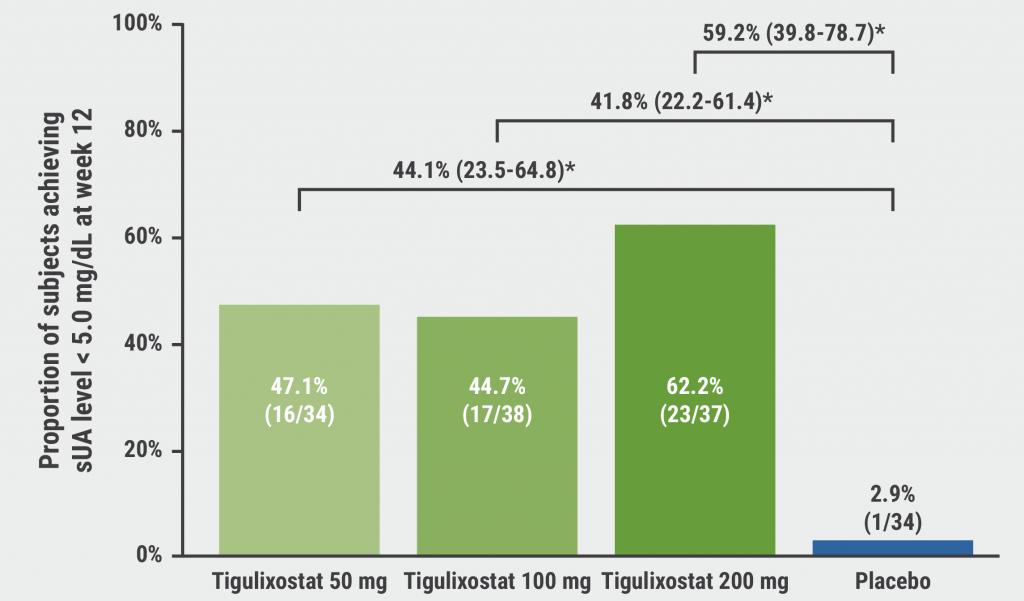
*Difference in proportion (%) to placebo with P<0.0001; asymptotic limits of 95% CI and risk difference were determined by the Wald method.
As for safety, 50–56.8% on tigulixostat and 50% on placebo were affected by treatment-emergent adverse events, the majority of mild-to-moderate severity; 4 patients discontinued the study due to adverse events. Between 10.8% and 13.2% of tigulixostat patients and 9.4% of placebo patients experienced a gout flare that required rescue treatment.
“In conclusion, tigulixostat significantly lowered serum urate in gout patients, impressively achieved urate targets, and was well tolerated collectively. These results support its continued development for gout with uncontrolled hyperuricemia,” Prof. Terkeltaub summarised.
- Terkeltaub R. Phase 2 study results from a randomised, double-blind, placebo-controlled, dose-finding study to evaluate efficacy and safety of tigulixostat, a novel non-purine selective xanthine oxidase inhibitor in gout patients with hyperuricemia. Abstract L05, ACR Convergence 2021, 3–10 November.
- Mu Z, et al. Clin Rheumatol. 2019 Dec;38(12):3511-3519.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Laboratory and clinical signs 24h after hospitalisation predict MIS-C in children Next Article
Lupus patients less protected by COVID-19 vaccine »
« Laboratory and clinical signs 24h after hospitalisation predict MIS-C in children Next Article
Lupus patients less protected by COVID-19 vaccine »
Table of Contents: ACR 2021
Featured articles
Late-Breaking Abstracts
Vaccine booster improves immune response in patients treated with rituximab
IL-17 inhibition showing efficacy in GCA in phase 2 trials
Spotlight on Rheumatoid Arthritis
Cycling JAK inhibitors shows similar effectiveness to switching to a bDMARD in difficult-to-treat RA
Pre-existing heart failure affects safety of hydroxychloroquine in RA patients
Patients with RA-associated interstitial lung disease benefit from antifibrotic agent
Ultra-low dosing of rituximab in RA is a viable treatment option
Kidney disease and hydroxychloroquine dose are risk factors for developing retinopathy
More pros than cons for the use of statins in RA
Psoriatic Arthritis: Novel Developments
Selective IL-23 inhibition: a new option in active PsA
Ustekinumab: highly efficacious in PSA independent of methotrexate
COVID-19: What You Need to Know
Vaccinated rheumatic patients carry increased risk for COVID-19 breakthrough infections
B-cell depleting medication increases COVID-19 breakthrough infection outcome risk
COVID-19 mRNA vaccine safe and tolerable in adults with autoimmune disease
SLE Treatment: What Is New
Iberdomide: an upcoming new treatment possibility in lupus erythematosus
Sequential rituximab after belimumab does not improve disease control in SLE
Lupus patients less protected by COVID-19 vaccine
Late-Breaking Posters
Promising results in uric acid-lowering in gout patients with a new xanthine oxidase inhibitor
Laboratory and clinical signs 24h after hospitalisation predict MIS-C in children
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

