COPD is a leading cause of death worldwide. An increase of 17.5% was seen in deaths in 2017 compared with 2007. Despite progress in the vaccination field, a vaccine against the most frequently detected bacteria associated with acute exacerbation in COPD patients has not been licensed yet. Acute exacerbations in COPD are heterogenous in nature. Bacterial infections with non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) are frequently associated with acute exacerbations. Prof. Stefan Andreas (Gastarzt Abteilung Pneumologie der Medizinischen Klinik II Gießen, Germany) and his team have been investigating a candidate NTHi-Mcat vaccine containing bacterial surface proteins [1].
The aim of the current randomised, placebo-controlled, observer-blind, multicentre trial was to investigate whether vaccination reduces the number of exacerbations in patients with COPD. In the trial, 67 sites were recruiting patients, mainly in Europe but also in the United Kingdom and the United States. The most important inclusion criterium was having at least 1 moderate or severe acute exacerbation of COPD in the last 12 months. Patients were randomised to receive placebo or the vaccine containing NTHi and Mcat antigens combined with a liposome-based adjuvant, added to create a stronger immune response. Two intermuscular injections of vaccine or placebo were given 60 days apart. Sputum and blood samples were collected for immunogenicity assessment. Quantitative PCR was done to detect bacteria in sputum. The primary outcome of the study was the rate of moderate or severe exacerbations 1 year after the vaccination period, starting 1 month after the 2-dose vaccination.
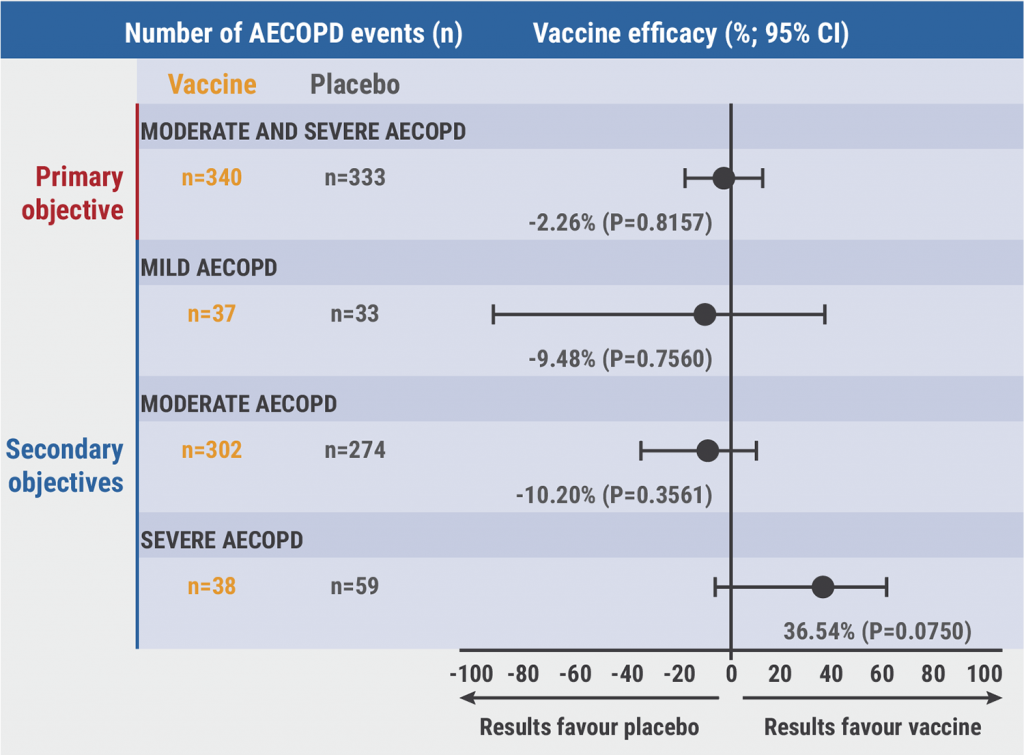
In total, 673 COPD patients were included in the study: 340 patients in the vaccine group and 333 patients in the placebo group. No vaccine efficacy was shown for moderate and severe acute exacerbations of COPD, mild acute exacerbations of COPD, and moderate acute exacerbations of COPD (P=0.83, P=0.76, P=0.37, respectively; see Figure 1). However, severe acute exacerbations of COPD (n=38 vaccine group; n=59 control group) were reduced by 36.7% (P=0.07).
Figure 1: Vaccine efficacy data [1]
No safety concerns were identified. Only a small number of severe adverse events and no vaccine-related serious adverse events were reported. Local adverse events as mild pain, redness and/or swelling at the injection side were common. Immunogenicity results showed that the candidate vaccine induced an antigen-specific immune response (see Figure 2).
Figure 2: Vaccine immunogenicity data [1]

Taken together, the primary endpoint of reducing the frequency of moderate and severe acute exacerbations of COPD was not met. However, immunogenicity and vaccine efficacy data looked promising as a reduction of severe acute exacerbations of COPD was demonstrated. The presented study was the first randomised controlled trial to investigate a vaccine against two frequently detected bacteria in COPD patients. The data may encourage further investigation of the candidate NTHi-Mcat vaccine.
- Andreas S, et al. Late Breaking Abstract - First-time assessment of efficacy of candidate vaccine to prevent acute exacerbations of chronic obstructive pulmonary disease (AECOPD): multicentre, randomised, controlled, observer-blind phase 2b trial. Abstract 210. ERS 2021, 5–8 September.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« The effect of the pandemic on the discharge diagnosis of older COPD patients Next Article
COPD symptoms and lung function related to sleep quality »
« The effect of the pandemic on the discharge diagnosis of older COPD patients Next Article
COPD symptoms and lung function related to sleep quality »
Table of Contents: ERS 2021
Featured articles
Letter from the Editor
COVID-19 Research: Looking Back and Moving Forward
Higher inflammation markers in COVID-19 patients with a first negative PCR test
Persistent fatigue following COVID-19
Risk of COVID-19-related morbidity and mortality in young and middle-aged adults
Respiratory Viral Infections: Insights from Recent Studies
Rhinovirus bronchiolitis increased risk of recurrent wheezing and asthma
COPD: Evidence Update
Livestock farming affected the airway microbiome of COPD patients
Reduction of COPD severe acute exacerbations by candidate vaccine
Paediatrics and Vaccinology
Better lung function in children with a healthy diet
Need for validated severity score in the assessment of bronchiolitis
Increased impact of air pollution on lung function in preterm infants
Pearls in Asthma Research
Biomarkers do not discriminate severe from severe uncontrolled asthma
Increased blood neutrophiles in patients with obesity and asthma
Blood inflammatory phenotypes associated with clinical symptoms of asthma
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

