https://doi.org/10.55788/f85f00cc
Results from the phase 1b KEYNOTE-173 and the phase 2 I-SPY 2 studies already demonstrated that the combination of pembrolizumab with chemotherapy as neoadjuvant treatment had manageable side effects and promising anti-tumour activity in patients with locally advanced triple-negative breast cancer [1,2]. The phase 3 KEYNOTE-522 study assessed pembrolizumab + chemotherapy (n=401) versus placebo + chemotherapy (n=201) as neoadjuvant therapy, followed by pembrolizumab versus placebo as adjuvant treatment in patients with early triple-negative breast cancer [3]. Key eligibility criteria were age ≥18 years, newly diagnosed triple-negative breast cancer of either T1c N1-2 or T2-4 N0-2, ECOG performance status 0-1, and availability of a tissue sample for programmed death-ligand 1 (PD-L1) assessment. Primary endpoints were pCR (ypT0/Tis ypN0) assessed by a local pathologist in intention-to-treat (ITT), and event-free survival (EFS) assessed by an investigator in ITT. Secondary endpoints of the study were pCR as per alternative definitions (ypT0 ypN0 and ypT0/Tis), overall survival (OS), pCR, EFS, and OS in the PD-L1–positive population, and safety in all treated patients. Exploratory endpoints were residual cancer burden (RCB), pCR by patient subgroups, EFS by pCR, and EFS by tumour infiltrating lymphocytes (TILs). A subgroup analysis of patients with lymph node involvement was performed to assess the effect of adding pembrolizumab to neoadjuvant chemotherapy and adjuvant therapy in patients with early-stage triple-negative breast cancer.
The results showed that 64.8% of patients with lymph node involvement who received pembrolizumab + chemotherapy had a pCR versus 51.2% of patients who were treated with chemotherapy alone (difference 13.6; range 5.4–21.8; P=0.00055). The first pre-planned interim analysis for EFS based on 1,174 patients had a pre-calculated P-value boundary for significance of 0.000051 (HR<0.4); median follow-up was 15.5 months. It showed that 91.3% of patients receiving pembrolizumab + chemotherapy achieved EFS versus 85.3% of patients receiving chemotherapy. It was also reported that patients with stage 3 disease had high rates of pCR (see Figure).
Figure. Pathologic complete response by disease stage [3]
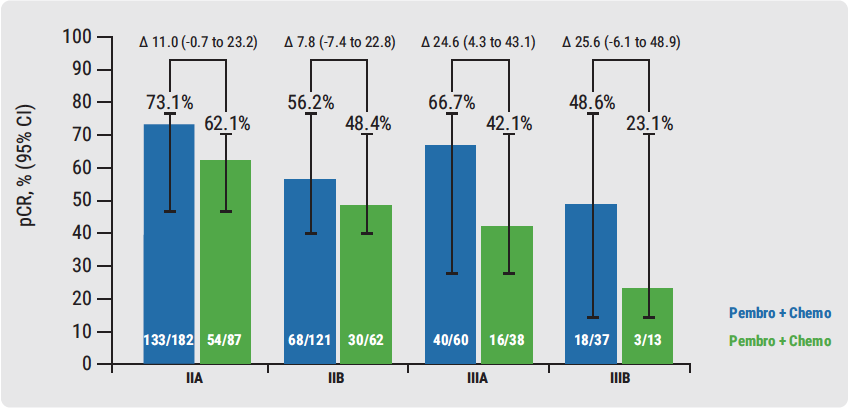
Immune-mediated adverse event rates were consistent with the known iles of each regimen and represented no new safety concern. Researchers suggest that adding pembrolizumab to neoadjuvant chemotherapy is beneficial for patients with the most aggressive disease and the highest unmet need. These findings may even be practice-changing. Further follow-up is needed to confirm EFS benefit and the long-term safety profile, and additional biomarker analyses including TILs and BRCA are planned as well.
1. Schmid P, et al. J Clin Oncol. 2017;35:556-556.
2. Nanda R, et al. J Clin Oncol. 2017;35;506-506.
3. Schmid P, et al. GS3-03. SABCS 2019.
Posted on
Previous Article
« HER2 antibody-drug conjugate T-DXd promising in T-DM1 pretreated patients Next Article
Breast cancer incidence decreased by oestrogen alone but increased by adding progestin »
« HER2 antibody-drug conjugate T-DXd promising in T-DM1 pretreated patients Next Article
Breast cancer incidence decreased by oestrogen alone but increased by adding progestin »
Table of Contents: SABCS 2019
Featured articles
Screening, Detection, and Diagnosis
Phase 2 Trial Update
Phase 3 Trial Update
Long-Term Study Results
Triple-Negative Breast Cancer
HER2-Positive Breast Cancer
Related Articles
February 13, 2020
Subcutaneous pertuzumab + trastuzumab non-inferior to the IV combination
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

