https://doi.org/10.55788/6101c6da
Previously, results of the phase 3 CheckMate 227 trial (NCT02477826) showed that first-line dual immune blockade with nivolumab plus ipilimumab provides a durable, long-term OS benefit versus chemotherapy in patients with advanced NSCLC, regardless of PD-L1 expression or histology [1].
The phase 3 KEYNOTE-598 trial (NCT03302234) evaluated the efficacy and toxicity of first-line pembrolizumab plus ipilimumab versus pembrolizumab monotherapy in patients with metastatic NSCLC with PD-L1 tumour proportion score (TPS) ≥50%. In the protocol-specified interim analysis, it was concluded that the addition of ipilimumab to pembrolizumab did not improve OS or progression-free survival (PFS) [2]. As a consequence, ipilimumab was discontinued and all patients continued with pembrolizumab monotherapy. Dr Delvys Rodriguez-Abreu (Universidad de Las Palmas de Gran Canaria, Spain) presented the 3-year follow-up results [3].
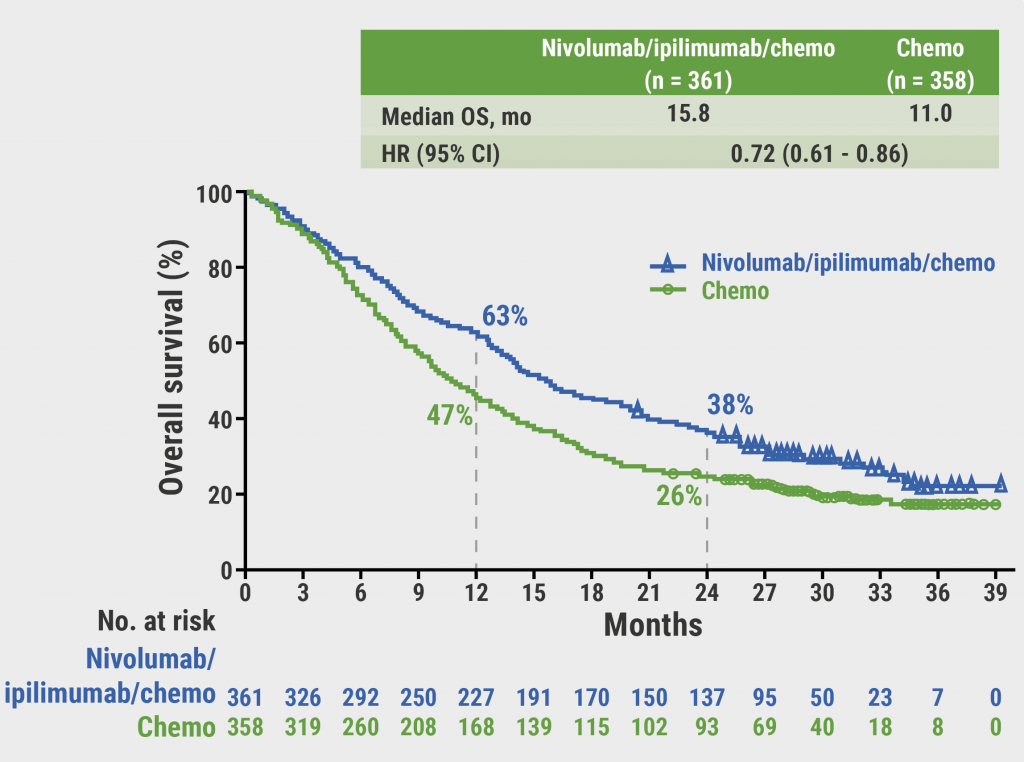
In KEYNOTE-598, a total of 568 patients with previously untreated, stage IV NSCLC and PD-L1 TPS ≥50% were randomised 1:1 to pembrolizumab plus ipilimumab or pembrolizumab plus placebo. Ipilimumab and placebo were given up to 18 doses; pembrolizumab up to 35 doses. Dual primary endpoints were OS and PFS. The median time from randomisation to data cut-off was 33.6 months.
After discontinuing ipilimumab or placebo for all patients, median OS and PFS remained similar between groups. Median OS was 22.1 and 22.7 months, and median PFS was 8.2 and 8.4 months, respectively, in the pembrolizumab plus ipilimumab and pembrolizumab plus placebo arms. Two-year OS rate was 48.0% and 48.5%, respectively; 2-year PFS rate was 27.2% and 25.1%, respectively. Objective response rate (ORR) was 46.5% and 46.1%, respectively.
Among participants initially treated with pembrolizumab plus ipilimumab (n=52) versus pembrolizumab plus placebo (n=71) who completed 35 cycles of pembrolizumab, ORR was 88.5% versus 87.3%, respectively; OS and PFS rates 1 year from completing pembrolizumab were 86.3% versus 87.6% and 72.8% versus 83.5%, respectively.
Grade 3–5 treatment-related adverse events occurred in 35.1% and 20.3% of participants in the pembrolizumab plus ipilimumab and pembrolizumab plus placebo arm, respectively.
Overall, with continued follow-up, results did not show a survival benefit with pembrolizumab plus ipilimumab versus pembrolizumab monotherapy. Two-year OS rates were similar between groups and safety favoured pembrolizumab monotherapy. “Therefore, pembrolizumab monotherapy remains a standard of care therapy for metastatic NSCLC with PD-L1 TPS ≥50%,” concluded Dr Rodriguez-Abreu.
- Paz-Ares LG, et al. Abstract 9016, ASCO Annual Meeting 2021, 04–08 June.
- Boyer M, et al. J Clin Oncol. 2021;39:2327–2338.
- Rodriguez-Abreu D, et al. Pembrolizumab plus ipilimumab or placebo in previously untreated metastatic NSCLC with PD-L1 tumor proportion score ≥50%: KEYNOTE-598 3-year follow-up. Abstract 6MO. ELCC 2022 Virtual Meeting, 30 March–02 April.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« In concurrent CRT for stage III, unresectable NSCLC, performance status is better with proton therapy than photon therapy Next Article
Updated results of CameL-sq trial confirm benefit of camrelizumab »
« In concurrent CRT for stage III, unresectable NSCLC, performance status is better with proton therapy than photon therapy Next Article
Updated results of CameL-sq trial confirm benefit of camrelizumab »
Table of Contents: ELCC 2022
Featured articles
Early-Stage Non-Small Cell Lung Cancer
Real-world treatment and survival in early-stage NSCLC
Consistent efficacy of osimertinib in Chinese and global population
Promising efficacy of neoadjuvant osimertinib in EGFR-mutated NSCLC
Peri-operative survival in bilobectomy is comparable with that of left pneumonectomy
Advanced Non-Small Cell Lung Cancer
Pro-inflammatory tumour profile predicts complete pathological response to neoadjuvant chemoimmunotherapy
Furmonertinib outperforms gefitinib as first-line therapy in patients with EGFR-mutated NSCLC
Second-line oritinib demonstrated potential clinical benefit in advanced EGFR-mutated NSCLC
Updated results confirm efficacy and safety of entrectinib in patients with NTRK fusion-positive NSCLC
ROS1 rearrangement-targeting unecritinib is a potential new first-line strategy
Savolitinib is effective in patients with MET-mutated NSCLC
Sintilimab plus chemotherapy improves OS in treatment-naïve, stage III–IV non-squamous NSCLC
Updated results of CameL-sq trial confirm benefit of camrelizumab
No long-term benefit of adding ipilimumab to pembrolizumab in metastatic NSCLC
In concurrent CRT for stage III, unresectable NSCLC, performance status is better with proton therapy than photon therapy
No improved prognosis for concurrent versus sequential immune checkpoint inhibition and CRT in unresectable NSCLC
Durvalumab after sequential CRT safe in stage III, unresectable NSCLC
No impact of grade ≥2 pneumonitis on patient-reported outcomes in PACIFIC
Immunotherapy delays deterioration in health-related quality of life in metastatic NSCLC
Small Cell Lung Cancer
Total metabolic tumour volume: a new potential prognostic factor in SCLC
Radiation dose on oesophagus predicts OS in SCLC patients treated with chemoradiotherapy
Characteristics of long-term survivors in the CASPIAN trial
Outcomes of real-world CANTABRICO trial match results from CASPIAN
Lung Cancer Epidemiology
Lung cancer diagnosis with liquid biopsy of peripheral blood cells
Rare EGFR mutations as oncogenic drivers
Decline in lung cancer mortality is almost exclusive to men
Related Articles
November 8, 2019
New technologies in lung cancer detection
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

