https://doi.org/10.55788/3f0abdc4
Non-oral acute therapies for migraine are recommended when oral forms are associated with inadequate response, slow onset of action, or poor tolerability. Rapid onset effect is a priority for many patients with migraine, said Prof. Richard Lipton (Albert Einstein College of Medicine, NY, USA), who presented the study results [1]. He added that most migraine patients prefer nasal sprays to injectables. Zavegepant is the only small-molecule calcitonin gene-related peptide (CGRP) receptor antagonist delivered by nasal spray in late-stage development for the acute treatment of migraine. Its effects and safety were compared with a placebo in a phase 3, double-blind, randomised trial (NCT04571060).
The participants were adults with typically 2–8 moderate or severe monthly migraine attacks. They self-administered 1 dose of 10 mg zavegepant nasal spray or a matching placebo to treat 1 migraine attack. The coprimary efficacy endpoints were freedom from pain and freedom from the most bothersome symptom 2 hours after the intervention.
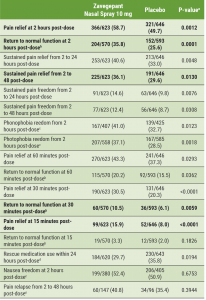
Participants had a mean age of 41 years; 83% were women. The most bothersome symptom was photophobia in 60.4%, nausea in 24.7%, and phonophobia in 15.0%. A total of 1,269 participants were evaluable for efficacy. Prof. Lipton said that zavegepant nasal spray relieved pain as early as 15 minutes post-dose (15.9% vs 8.0%; P<0.0001). Within 2 hours of administration, significantly more patients achieved freedom from pain (23.6% vs 14.9%; P<0.0001). Zavegepant was superior to the placebo in 13 prespecified secondary endpoints (see Table). Among these endpoints, Prof. Lipton highlighted a return to normal function after 2 hours (“a highly significant difference”) and sustained pain relief 15 minutes post-dose, both of which are highly valued by patients.
Table: Secondary efficacy endpoints in a pre-specified hierarchical order [1]
Most adverse events were mild or moderate; none were serious. The most common adverse events were dysgeusia (20.5% vs 4.7%), nasal discomfort (3.7% vs 0.8%), and nausea (3.2% vs 1.1%). There was no signal of hepatoxicity.
Additional trials are needed to establish the long-term safety and consistency of effect across attacks. In conclusion, Prof. Lipton said: “It is exciting to now have a non-oral option for migraine patients who benefit from the class of CGRP receptor antagonists.”
- Mullin K. Efficacy and safety of zavegepant nasal spray for the acute treatment of migraine: Results of a Phase 3 double-blind, randomized, placebo controlled trial. PL5.002, AAN 2023 Annual Meeting, 22–27 April, Boston, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Atogepant helps prevent treatment-resistant episodic migraine Next Article
Lecture on migraine: from the prodromal phase to future paradigm shifts »
« Atogepant helps prevent treatment-resistant episodic migraine Next Article
Lecture on migraine: from the prodromal phase to future paradigm shifts »
Table of Contents: AAN 2023
Featured articles
Letter from the Editor
Positive results for hereditary transthyretin-mediated amyloid polyneuropathy
Infectious Diseases
Allogenic T-cell-based immunotherapy for PML in development
Cognitive Impairment and Dementia
Lecanemab may slow decline of cognition and function in Alzheimer’s Disease
Donanemab shows rapid and deep plaque clearance in early Alzheimer’s Disease
Epilepsy
Seizure forecasting and detection with wearable devices are feasible
Encouraging first results of GABAergic interneurons implants for focal epilepsy
Headache and Migraine
Lecture on migraine: from the prodromal phase to future paradigm shifts
Zavegepant nasal spray exhibits good efficacy and safety in acute migraine
A vaccine as a potentially safe and effective immunotherapy against CGRP
Multiple Sclerosis
Teriflunomide prevents conversion to MS in patients with RIS
Gold nanocrystals may be effective as adjunctive MS therapy
Muscle and Neuro-Muscular Disorders
First-ever ALS platform trial reports on outcomes of 4 treatments
Pridopidine for Huntington’s disease fails to meet the primary endpoint
Parkinson's Disease
Continuous levodopa/carbidopa infusion shows favourable safety and efficacy
Unilateral right STN-DBS improves verbal fluency
Stroke
Harnessing the microbiome as a possible stroke treatment
Patients with a large core infarct benefit from thrombectomy
Miscellaneous
Artificial intelligence applications in neurology: seize the moment
Spinal cord stimulation eases painful diabetic neuropathy
EVT improves functional outcomes in Chinese patients with BAO
Severe sleep apnoea associated with white matter hyperintensities
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

