Treatment options for patients with R/R DLBCL have increased in recent years. Tafasitamab in combination with lenalidomide is approved for adult patients with R/R DLBCL not otherwise specified, including patients with DLBCL arising from low-grade lymphoma, and patients who are ineligible for ASCT.
Efficacy of the chemotherapy-free regimen tafasitamab plus lenalidomide in this patient population was demonstrated in the single-arm, phase 2 L-MIND study (NCT02399085). The overall response rate (ORR) was 57.5% in this trial. “Responses were quite durable,” Dr Grzegorz Nowakowski (Mayo Clinic, Rochester, USA) commented on these results. Median duration of response (DoR) was 43.9 months, median progression-free survival (PFS) was 11.6 months, and median overall survival (OS) was 33.5 months [2,3].
Lack of head-to-head comparisons
Assessing comparative effectiveness of novel treatments in randomised head-to-head studies is time-consuming, costly and may delay patient access to new treatment options. In the absence of randomised clinical trials, real-world data can be used to generate external comparators to complement single-arm clinical trials.
RE-MIND2 (NCT04697160) compared patient outcomes from L-MIND with matched patient populations treated with NCCN/ESMO recommended therapies for ASCT‑ineligible patients with R/R DLBCL. Dr Nowakowski presented results from an expanded analysis of RE-MIND2 comparing tafasitamab plus lenalidomide compared with polatuzumab vedotin, bendamustine plus rituximab (pola-BR), rituximab plus lenalidomide (R2), and CD19 CAR T-cell therapies (CAR T).
OS benefit
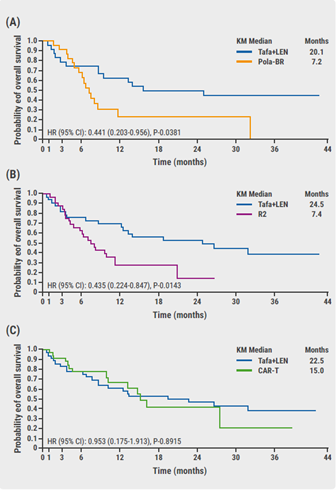
Data of almost 3,500 patients was retrospectively collected. The primary endpoint was OS. A significant OS benefit was associated with tafasitamab plus lenalidomide compared with pola-BR (HR 0.44, 95% CI 0.20–0.96; P=0.038) and R2 (HR 0.44; 95% CI 0.22–0.84; P=0.014) (Figure 1A–B). In contrast, no significant difference in OS benefit between tafasitamab plus lenalidomide and CAR T was found (HR 0.95; 95% CI 0.47–1.91; P=0.891) (Figure 1C).
Secondary endpoints included objective response rate (ORR), complete response rate, PFS and DoR. ORR was 62.5% for tafasitamab plus lenalidomide versus 58.3% for pola-BR (P=1.000), 63.6% versus 30.3% for R2 (P=0.013), and 59.5% versus 75.7% for CAR T (P=0.214). Improved outcomes were also observed with tafasitamab plus lenalidomide for other secondary endpoints. Results were consistent with those obtained in the sensitivity analyses.
Conclusions
Tafasitamab plus lenalidomide improved survival outcomes compared with pola-BR and R2 in closely matched patient populations. “We found statistically significant and clinically meaningful improvements in median OS for patients receiving tafasitamab plus lenalidomide,” Dr Nowakowski concluded. “Median OS in patients who received tafasitamab plus lenalidomide was comparable to the median OS in patients who received CAR T therapies.” In addition, numerical differences favouring tafasitamab plus lenalidomide were observed for the secondary endpoints. Sensitivity analyses confirmed the main analysis.
The RE-MIND2 study design used strict patient-level matching to compare real-world and clinical trial populations. This allows for a contextualisation of outcomes with different treatments in the absence of head-to-head trials. Because of the recent approval of comparator treatments, this data may help to make informed treatment decisions about R/R DLBCL therapies.
- Nowakowski G, et al. Tafasitamab Plus Lenalidomide Versus Pola BR, R2, and CAR T: Comparing Outcomes from RE-MIND2, an Observational, Retrospective Cohort Study in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Abstract 183, ASH 2021 Annual Meeting, 11-14 Dec.
- Salles G, et al. Lancet Oncol. 2020;21:978-988.
- Duell J, et al. Haematologica. 2021;106:2417-2426.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Axi-cel more effective but tisa-cel less toxic in DLBCL Next Article
Axi-cel improved event-free survival in R/R DLBCL »
« Axi-cel more effective but tisa-cel less toxic in DLBCL Next Article
Axi-cel improved event-free survival in R/R DLBCL »
Table of Contents: ASH 2021 Focus on CAR T-Cell Therapy
Featured articles
Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma
CAR T-cell Therapy
Most re-hospitalisations within first month from CAR T-cell infusion
CD22-directed CAR T-cell therapy safe and well-tolerated in R/R LBCL
High rate of rapid and complete responses with axi-cel in high-risk large B-cell lymphoma
Novel anti-CD19 plus lenalidomide prolonged survival in R/R DLBCL
Liso-cel superior to standard-of-care as second-line therapy in large B-cell lymphoma
CIRS is predictive of outcomes in CAR T-cell recipients with R/R DLBCL
Axi-cel more effective but tisa-cel less toxic in large B-cell lymphoma
Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma
Comparable outcomes with second-line tisa-cel versus standard-of-care for relapsed/refractory aggressive NHL
Improved QoL with axi-cel versus standard-of-care in R/R LBCL
Related Articles
February 9, 2021
Anti-CD20 therapy for lymphoma tied to worse COVID-19 outcomes
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

