Patients with LBCL who relapse early or are refractory to first-line therapy often experience poor outcomes. In addition, patients receiving second-line standard-of-care treatment frequently report poor health-related QoL [2].
Axi-cel is an autologous anti‑CD19 chimeric antigen receptor (CAR) T-cell therapy, approved for patients with R/R LBCL after ≥2 lines of therapy. In the open-label ZUMA-7 study, axi-cel was compared with standard-of-care in the second line. A long-term follow-up analysis of ZUMA-1, which was presented at ASH 2021, demonstrated a 5-year overall survival (OS) rate of 43% after a median follow-up of 63 months for axi-cel in patients with refractory LBCL [3]. Dr Mahmoud Elsawy (Dalhousie University, Halifax, USA) and others conducted the first comparative analysis of CAR T-cell therapy (as second-line treatment) versus standard-of-care on PROs in this patient population.
Improved QoL
Of the 359 enrolled patients, 296 patients had completed PROs at baseline, as well as and ≥1 follow-up measure(s), and were included for analysis. Overall, 70% of the patients had primary refractory disease, 42% had high second-line age-adjusted International Prognostic Index (2–3), and 30% were ≥65 years old. Patients treated with axi-cel showed a statistically significant increase in QoL compared with patients receiving standard-of-care (P<0.0001) and a clinically meaningful difference at day 100 in mean change of scores from baseline on all prespecified PRO domains (see Figure).
Figure: Change from baseline for prespecified PRO endpoints
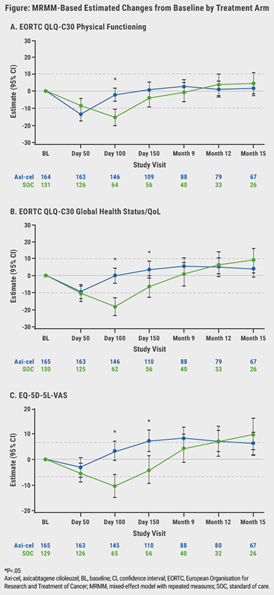
These results were confirmed in sensitivity analyses, wherein significance was retained at day 100. Furthermore, also other QoL measures favoured axi-cel over standard-of-care, as indicated by the EORTC QLQ-C30 Global Health Status/QoL (P=0.0124) and EQ-5D-5L VAS (P=0.0004).
According to Dr Elsawy, another important finding was that the mean estimated scores for the axi-cel arm had numerically returned to or exceeded the baseline scores at day 150 compared with the scores of the standard-of-care arm. “This suggests that patients receiving axi-cel return faster to their baseline QoL level compared to those receiving standard-of-care.”
Attrition and additional exploratory analyses
Attrition, for example due to disease progression, new lymphoma therapy, or death, was substantial after Month 9. Attrition was disproportionally higher in the standard-of-care arm and this may have caused biases in patient population. Therefore, score comparisons at later timepoints warrant cautious interpretation.
Additional exploratory analyses of PRO endpoints, such as EORTC QLQ-C30 role functioning, social functioning, fatigue, nausea/vomiting, dyspnoea, insomnia, appetite loss, diarrhoea, and EQ-5D-5L index, clearly showed improvements with axi-cel compared with standard-of-care.
Conclusion
The superior clinical outcomes and patient experiences with axi-cel over standard-of-care may help physicians make informed decisions about the second-line treatment of patients with R/R LBCL.
- Elsawy M, et al. Patient-Reported Outcomes in a Phase 3, Randomized, Open-Label Study Evaluating the Efficacy of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard of Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma (ZUMA-7). 430, ASH 2021 Annual Meeting, 11-14 Dec.
- Lin V, et al. J Clin Oncol. 2020;38(15_suppl):e20070.
- Jacobson CA, et al. Abstract 1764, ASH 2021 Annual Meeting, 11-14 Dec.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Dapagliflozin improves stroke volume in patients with HFrEF Next Article
Comparable outcomes with second-line tisa-cel versus standard-of-care for relapsed/refractory aggressive NHL »
« Dapagliflozin improves stroke volume in patients with HFrEF Next Article
Comparable outcomes with second-line tisa-cel versus standard-of-care for relapsed/refractory aggressive NHL »
Table of Contents: ASH 2021 Focus on CAR T-Cell Therapy
Featured articles
Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma
CAR T-cell Therapy
Most re-hospitalisations within first month from CAR T-cell infusion
CD22-directed CAR T-cell therapy safe and well-tolerated in R/R LBCL
High rate of rapid and complete responses with axi-cel in high-risk large B-cell lymphoma
Novel anti-CD19 plus lenalidomide prolonged survival in R/R DLBCL
Liso-cel superior to standard-of-care as second-line therapy in large B-cell lymphoma
CIRS is predictive of outcomes in CAR T-cell recipients with R/R DLBCL
Axi-cel more effective but tisa-cel less toxic in large B-cell lymphoma
Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma
Comparable outcomes with second-line tisa-cel versus standard-of-care for relapsed/refractory aggressive NHL
Improved QoL with axi-cel versus standard-of-care in R/R LBCL
Related Articles
September 24, 2021
Subcutaneous epcoritamab shows promise in some non-Hodgkin lymphoma patients

August 5, 2022
Novel first-line treatment option for mantle cell lymphoma
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

