The phase 2 GALAXI-1 trial (NCT03466411) randomised participants with moderately to severely active CD, who failed on prior conventional and/or biologic therapies, to 200 mg guselkumab, 600 mg guselkumab, 1,200 mg guselkumab, or placebo (intravenous, every 4 weeks). The results from the 12-week induction study demonstrated that guselkumab is associated with significantly improved clinical outcomes compared with placebo, according to Prof. Silvio Danese (Vita-Salute San Raffaele University, Italy) [1]. After week 12, participants in the 1,200 mg and 600 mg arms received 200 mg guselkumab, subcutaneous, every 4 weeks (n=61; n=63), whereas participants who were originally randomised to 200 mg guselkumab, received 100 mg guselkumab, subcutaneous, every 8 weeks (n=61). In addition, an ustekinumab reference arm was included (90 mg subcutaneous, every 8 weeks; n=63). At ECCO 2022 the 48-week results were presented, in which the placebo arm was not included.
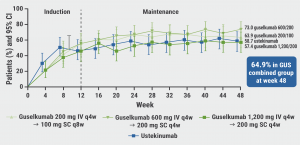
Of the participants on guselkumab, 64.9% achieved clinical remissiona through week 48 (see Figure), as compared with 58.7% in participants treated with ustekinumab. In addition, corticosteroid-free clinical remissionb proportions ranged between 55.7% and 71.4% in the guselkumab arms and was 58.7% in the ustekinumab arm. Similarly, Patient Reported Outcome (PRO-2) remissionc at week 48 was achieved by 50.8%– 69.8% of the participants treated with guselkumab and 46.0% of the participants treated with ustekinumab.
Figure: Participants in clinical remission through week 48 [1]
The proportion of serious adverse events (AEs) was 7.3% for the combined guselkumab arms and 12.7% for the ustekinumab arm. No opportunistic infections, cases of tuberculosis, or deaths were reported in any study group.
Prof. Danese mentioned that the current study was not powered to directly compare the different guselkumab arms in terms of efficacy. He also emphasised that the ustekinumab arm was a reference arm only. He expects guselkumab to be superior to ustekinumab in patients with CD, since the mechanism of action of guselkumab is more specific. However, a direct comparison needs to be performed between the 2 agents to prove this.
a. Clinical remission is defined as a Crohn's Disease Activity Index (CDAI) score <150.
b. Corticosteroid-free clinical remission is defined as a CDAI score <150 at week 48 and not receiving corticosteroids at week 48.
c. PRO-2 remission is defined as the unweighted CDAI component of daily abdominal pain score ≤1, and the unweighted CDAI component of daily average stool frequency score ≤3, and no worsening of abdominal pain or stool frequency from baseline.
- Danese S, et al. Clinical efficacy and safety of guselkumab maintenance therapy in patients with moderately to severely active Crohn’s Disease: Week 48 analyses from the phase 2 GALAXI 1 study. OP24, ECCO 2022, 16–19 February.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Mirikizumab efficacious for active ulcerative colitis Next Article
Guselkumab shows encouraging safety and efficacy in ulcerative colitis »
« Mirikizumab efficacious for active ulcerative colitis Next Article
Guselkumab shows encouraging safety and efficacy in ulcerative colitis »
Table of Contents: ECCO 2022
Featured articles
Upadacitinib maintenance therapy delivers sustained improvements in active ulcerative colitis
Novel Treatment Modalities
Guselkumab shows encouraging safety and efficacy in ulcerative colitis
Guselkumab maintenance therapy achieved high efficacy rates in Crohn’s disease
Mirikizumab efficacious for active ulcerative colitis
Risankizumab more efficacious in colonic than in ileal Crohn’s disease
Guselkumab plus golimumab promising combination for ulcerative colitis
Combined endpoint may support personalised medicine in ulcerative colitis
Filgotinib seems promising for perianal fistulising Crohn’s disease
Upadacitinib maintenance therapy delivers sustained improvements in active ulcerative colitis
Upadacitinib counters extraintestinal manifestations in ulcerative colitis
Deucravacitinib does not meet primary endpoint for ulcerative colitis
Head-to-Head Comparisons
Anti-TNFs versus vedolizumab and ustekinumab in Crohn’s disease
Upadacitinib appears to be an efficacious therapy for moderately-to-severely ulcerative colitis
Subcutaneous infliximab versus subcutaneous vedolizumab in IBD
Vedolizumab outperforms anti-TNF in biologic-naïve ulcerative colitis
Short-Term and Long-Term Treatment Results
Ozanimod treatment shows maintained response in ulcerative colitis
Stopping infliximab but not antimetabolites leads to more relapses in Crohn’s disease
Vedolizumab first approved therapy for chronic pouchitis
VEDOKIDS: Vedolizumab seems effective in paediatric IBD
Primary endpoint of 5-hydroxytryptophan for fatigue in IBD not met
Specific Therapeutic Strategies
Positive outcomes with therapeutic drug monitoring during infliximab maintenance therapy
Segmental colectomy beneficial over total colectomy in Chrohn’s disease
Modified 2-stage ileal pouch-anal anastomosis versus 3-stage alternative
Similar results for different corticosteroid tapering protocols in UC
Miscellaneous Topics
Lessons from the COVID-19 pandemic for IBD management
AI model distinguishes between histologic activity and remission in ulcerative colitis
Multi-Omic and dietary analysis of Crohn’s disease identifies pathogenetic factors
Novel classification system for perianal fistulising Crohn’s disease
Vaccination tool associated with improved vaccination coverage in IBD
Comparable safety profiles of biological therapies in elderly patients with IBD
Early biologic therapy induces larger effect than delayed treatment in Crohn’s disease
RESTORE-UC: No better outcomes with FMT superdonors than with autologous stools
Related Articles
November 2, 2020
Filgotinib effective as maintenance treatment for ulcerative colitis
December 1, 2022
Maintained symptom control with mirikizumab in UC
April 14, 2020
Subcutaneous ustekinumab as maintenance therapy in UC
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

