https://doi.org/10.55788/3517959b
“In Europe, CRC screening is inconsistent, and participation rates vary from 1% to 73%,” claimed Dr Julian Prosenz (Karl Landsteiner University of Health Sciences, Austria) [1]. “In Austria, there is opportunistic screening, but the uptake is unknown, and there is no mandatory quality assurance.” Dr Prosenz and co-investigators designed a study to investigate step-wise CRC screening among public healthcare provider employees between 50 and 65 years. The step-by-step screening process included an initial stool test (faecal immunochemical test [FIT] and M2 Pyruvate Kinase [M2PK]), and, if positive, a follow-up colonoscopy carried out by unselected endoscopists across the state. The study aimed to assess the performance and quality of this screening intervention.
In total, 10,239 employees were invited to join the screening (of which 74% were women) and 3,063 stool tests were analysed. The participation rate was higher among women (25%) than men (18%). In total, 747 stool tests turned out positive, 179 with a positive FIT test and 593 with a positive M2PK test. The initial acceptance rate for performing a follow-up colonoscopy was just below 80% (n=517). Most colonoscopies were performed by office-based physicians (66%), and the remaining were performed at a hospital (34%). Internists/gastroenterologists performed 59% of the procedures, and surgeons performed 41%.
No high-grade dysplasia or CRC was detected. The ADR was 20.5%, which is lower than the standard set by the European Society of Gastrointestinal Endoscopy (ESGE) for screening colonoscopy (25%). There were also differences in detection rates between office-based endoscopists (18.5%), and hospital endoscopists (24.3%). Furthermore, quality metrics such as Boston Bowel Preparation Scale (BBPS) >5 (88%), complete colonoscopy (85%), polyp size reported (48%), and PARIS/NICE/JNET classification reported (27%) did not meet the required ESGE standards (see Figure).
Figure: Quality metrics: study results versus required standards
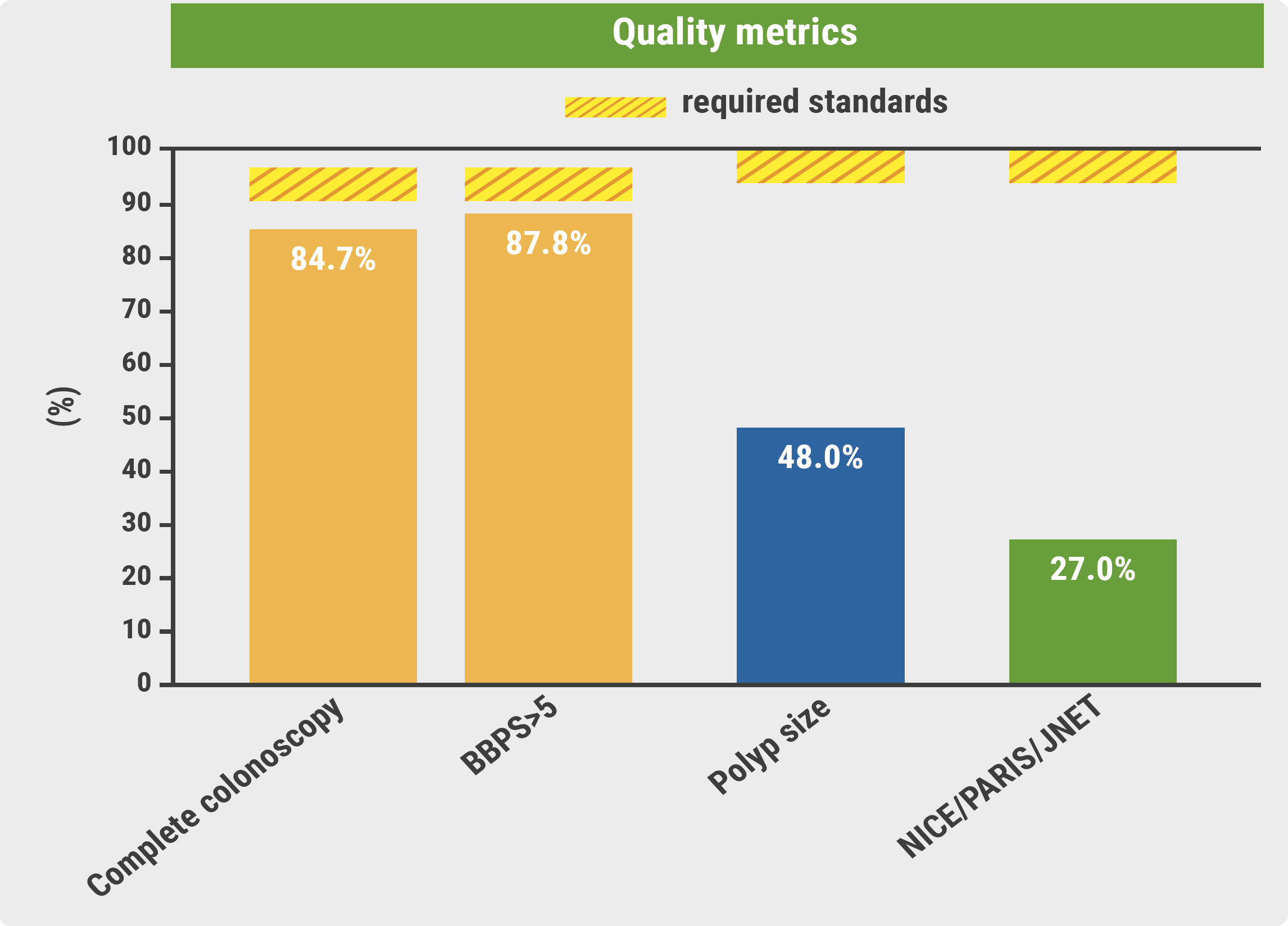
BBPS, Boston Bowel Preparation Scale.
In short, the current corporate-based CRC screening intervention yielded a participation rate of 23%, and 72% of the employees with a positive test underwent colonoscopy. “The participation rate is low, given that there was promotion of this study and repeated invitations,” added Dr Prosenz. Finally, the performance measures showed inconsistent quality and a wide variability in ADR and reporting standards.
- Prosenz J, et al. Results of a state-wide CRC screening initiative for 10,000 eligible health-care provider employees. LB09, UEG Week 2023, 14–17 October, Copenhagen, Denmark.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Epinephrine boosts efficiency in gastric ESD Next Article
How to deal with at-risk patients above the CRC screening age limit? »
« Epinephrine boosts efficiency in gastric ESD Next Article
How to deal with at-risk patients above the CRC screening age limit? »
Table of Contents: UEGW 2023
Featured articles
SEQUENCE: Risankizumab doubles endoscopic remission rates compared with ustekinumab in CD
What’s New in Artificial Intelligence
Digital intervention relieves symptoms and improves QoL in IBS
GastroGPT: Successful proof-of-concept study of gastroenterology-specific large language model
Other Therapeutics and Outcomes
Primary results from MAESTRO-NASH trial: resmetirom efficacious for NASH
Apraglutide: Advancing the treatment of short bowel syndrome
Endobiliary radiofrequency ablation in pCCA: a pilot study
Raising awareness for microscopic colitis: disease course and predictors
Outcomes of IBD Trials
DIVERSITY1: Filgotinib results in Crohn’s disease leave investigators puzzled
SEQUENCE: Risankizumab doubles endoscopic remission rates compared with ustekinumab in CD
Guselkumab provides benefits in UC regardless of advanced therapy history
INSPIRE: Risankizumab meets all efficacy endpoints in UC
Risankizumab resolves extraintestinal manifestations in CD
Obefazimod takes the spotlight as promising UC treatment
Rapid response to upadacitinib boosts outcomes in severe Crohn’s disease
LUCENT trials: Mirikizumab works in UC, regardless of targeted therapy history
ARTEMIS-UC: New kid in town for UC
Breakthroughs in Colorectal Lesions
Safer removal of large polyps with cold snare technique
Higher recurrence rates with cold snare EMR than with conventional EMR
How to deal with at-risk patients above the CRC screening age limit?
European CRC screening needs to be revised
Advances in Upper Endoscopy and Colonoscopy
Epinephrine boosts efficiency in gastric ESD
Artificial intelligence-aided colonoscopy did not improve outcomes in Lynch syndrome
Can computer technology improve our everyday colonoscopy results?
Is AI-assisted colonoscopy ready for clinical practice?
Should we use E-SEMS or EVT for traumatic oesophageal perforations?
Related Articles
December 7, 2023
Epinephrine boosts efficiency in gastric ESD
December 7, 2023
Is AI-assisted colonoscopy ready for clinical practice?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

