https://doi.org/10.55788/c0445527
In the U-EXCEED (NCT03345836) and U-EXCEL (NCT03345849) studies, upadacitinib demonstrated its efficacy compared with placebo as a treatment option for participants with moderately to severely active CD. Prof. Jean-Frederic Colombel (Icahn School of Medicine at Mount Sinai, NY, USA) presented a post-hoc analysis that concentrated on participants who demonstrated early clinical improvements within the initial 15 days of treatment [1]. These early responders were monitored and evaluated for clinical and endoscopic outcomes during the 12-week induction and 52-week maintenance phases.
At week 12, early responders exhibited higher rates of stool frequency and abdominal pain score (SF/APS) clinical remission (84% vs 46%) and mucosal healing (34% vs 21%) with upadacitinib compared with the overall population. These improvements persisted through the 52-week maintenance phase, underscoring the sustained effectiveness of upadacitinib (see Figure).
Figure: Clinical outcomes for early responders and the overall population at week 52 [1]
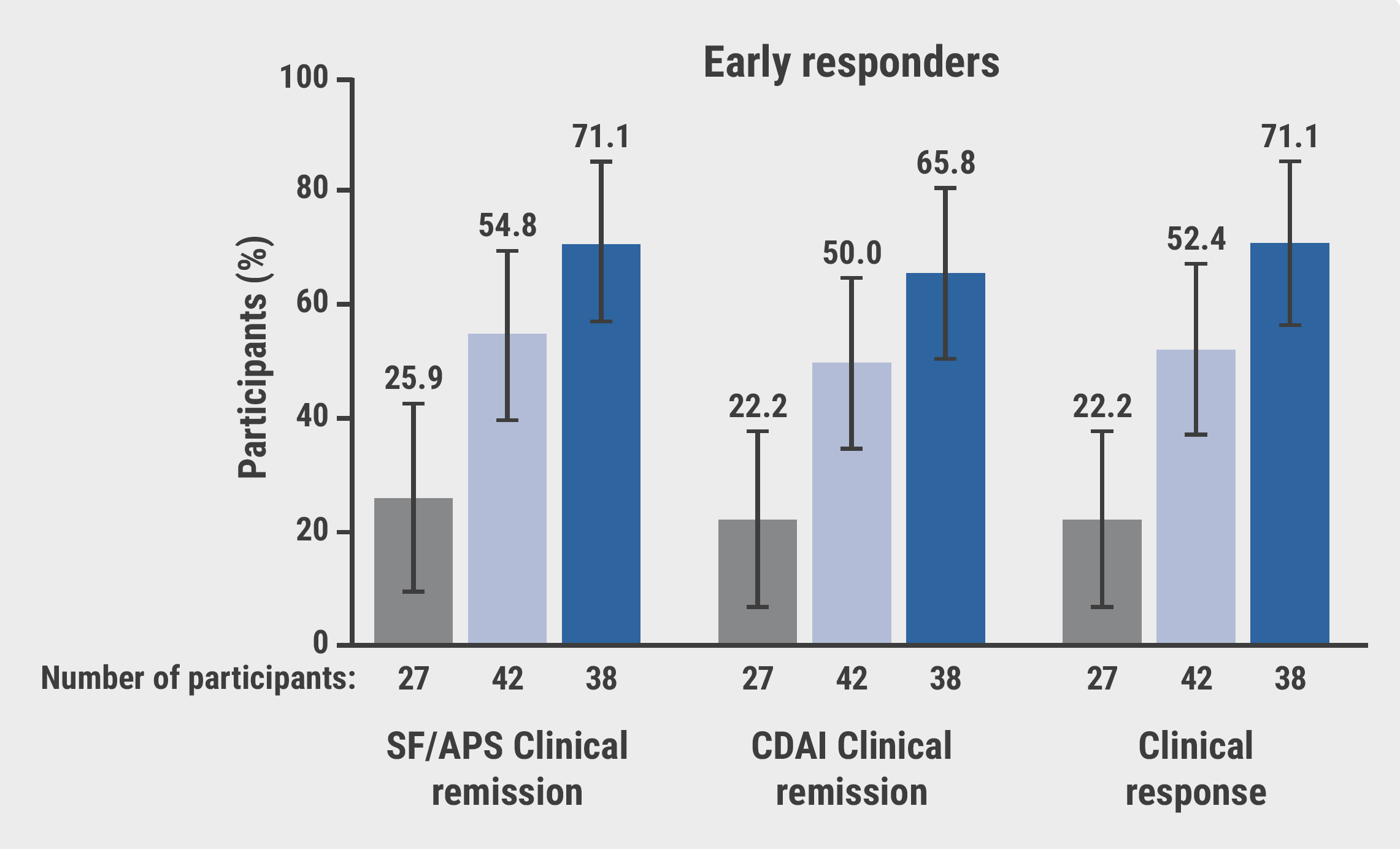
CDAI, Crohn’s Disease Activity Index; PBO, placebo; QD, once daily; SF/APS, stool frequency and abdominal pain score; UPA, upadacitinib.
"This is quite encouraging, and we should aim to look further at the predictors of response, as those patients who are responding very well to the treatment early on are doing well at weeks 12 and 52," Prof. Colombel emphasised. The findings suggest that an early response to upadacitinib therapy could be a strong indicator of sustained improvement and effectiveness in CD. This discovery could be instrumental in identifying and categorising patients more likely to benefit from upadacitinib therapy, enabling a more personalised and effective treatment approach.
- Colombel J-F, et al. Rapid clinical response to upadacitinib therapy is associated with improved clinical and endoscopic outcomes in patients with moderate to severe Crohn’s disease. OP099, UEG Week 2023, 14–17 October, Copenhagen, Denmark.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« LUCENT trials: Mirikizumab works in UC, regardless of targeted therapy history Next Article
Obefazimod takes the spotlight as promising UC treatment »
« LUCENT trials: Mirikizumab works in UC, regardless of targeted therapy history Next Article
Obefazimod takes the spotlight as promising UC treatment »
Table of Contents: UEGW 2023
Featured articles
SEQUENCE: Risankizumab doubles endoscopic remission rates compared with ustekinumab in CD
What’s New in Artificial Intelligence
Digital intervention relieves symptoms and improves QoL in IBS
GastroGPT: Successful proof-of-concept study of gastroenterology-specific large language model
Other Therapeutics and Outcomes
Primary results from MAESTRO-NASH trial: resmetirom efficacious for NASH
Apraglutide: Advancing the treatment of short bowel syndrome
Endobiliary radiofrequency ablation in pCCA: a pilot study
Raising awareness for microscopic colitis: disease course and predictors
Outcomes of IBD Trials
DIVERSITY1: Filgotinib results in Crohn’s disease leave investigators puzzled
SEQUENCE: Risankizumab doubles endoscopic remission rates compared with ustekinumab in CD
Guselkumab provides benefits in UC regardless of advanced therapy history
INSPIRE: Risankizumab meets all efficacy endpoints in UC
Risankizumab resolves extraintestinal manifestations in CD
Obefazimod takes the spotlight as promising UC treatment
Rapid response to upadacitinib boosts outcomes in severe Crohn’s disease
LUCENT trials: Mirikizumab works in UC, regardless of targeted therapy history
ARTEMIS-UC: New kid in town for UC
Breakthroughs in Colorectal Lesions
Safer removal of large polyps with cold snare technique
Higher recurrence rates with cold snare EMR than with conventional EMR
How to deal with at-risk patients above the CRC screening age limit?
European CRC screening needs to be revised
Advances in Upper Endoscopy and Colonoscopy
Epinephrine boosts efficiency in gastric ESD
Artificial intelligence-aided colonoscopy did not improve outcomes in Lynch syndrome
Can computer technology improve our everyday colonoscopy results?
Is AI-assisted colonoscopy ready for clinical practice?
Should we use E-SEMS or EVT for traumatic oesophageal perforations?
Related Articles
March 11, 2022
Robotic-assisted major liver resection may have advantages
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

