https://doi.org/10.55788/436c2478
In the treatment of CD, the efficacy and safety of upadacitinib have been demonstrated in the 2 induction studies U-EXCEED (NCT03345836) and U-EXCEL (NCT03345849). Responders at 12 or 24 weeks were eligible for re-randomisation and were included in the maintenance study U-ENDURE (NCT03345823). In these studies, the superiority of upadacitinib over placebo was demonstrated across clinical, endoscopic, steroid-free, and quality-of-life measures.
The aim of the sub-analysis presented by Dr Brian Feagan (University of Western Ontario, Canada) was to assess endoscopic outcomes in patients with or without a history of biologic failure, defined as inadequate response or intolerance, among those who received upadacitinib in the study programme. Pooled data from U-EXCEL, U-EXCEED, and U-ENDURE was used in this study. “In general, a lower inflammatory burden was seen in patients without biologic failure compared with those who failed a biologic,” Dr Feagan explained. In the biologic-failure group, 60% had at least failed 2 and many patients even 3 prior biologics, in most cases TNF blockers.
In the induction studies, endoscopic response (defined as a decrease in Simple Endoscopic Score for Crohn’s Disease [SES-CD] >50% from baseline or, for patients with an SES-CD of 4 at baseline, at least a 2-point reduction from baseline) and endoscopic remission (defined as SES-CD <4 and at least 2-point reduction from baseline and no subscore >1 in any individual variable) both showed higher rates in upadacitinib-treated participants compared with placebo. Of the participants treated with upadacitinib without biologic failure, 52% achieved an endoscopic response compared with 16.2% with placebo. This was 35.7% for upadacitinib-treated participants with prior biological failure compared with 5.3% on placebo. “In patients with prior biologic failure there was a lesser effect but still with a delta of 30,” Dr Feagan commented. At week 12, 36% of the upadacitinib-treated participants without biologic failure achieved endoscopic remission (vs 10.1% with placebo). The corresponding results for participants with biologic failure were 19.6% versus 2.8% in the placebo group.
The most difficult endpoint was sustained remission, which was defined as the percentage of participants who achieved endoscopic remission at both week 12 and 52. “Interestingly, patients with prior biologic failure had an enormous treatment effect,” Prof. Feagan said. Of participants with prior biologic failure, 60.3% achieved a sustained endoscopic response when treated with the high dose compared with 55% of participants without biologic failure. Endoscopic remission was achieved by 57.1% of participants treated with the high-dose upadacitinib and 35.5% treated with the low dose compared with 6.3% in the placebo group. Therefore, Dr Feagan pointed out that particularly patients with prior biologic failure have a better treatment effect with the higher dose.
Upadacitinib induction and maintenance treatment doses were well tolerated with no new safety signals. There were no deaths and there was a very low rate of opportunistic infections in the upadacitinib arm. In the 30 mg dose group, 1 embolic event was noted.
Superior symptom control in patients with UC
Upadacitinib also showed positive induction and maintenance results in UC. Prof. Geert D’Haens (Academic Medical Center University of Amsterdam, the Netherlands) presented an analysis of the phase 3 induction trials U-ACHIEVE (NCT02819635) and U-ACCOMPLISH (NCT03653026) and of the upadacitinib UC trial programme where they looked at complete symptom resolution defined as no bowel urgency, no abdominal pain, no rectal bleeding, and a stool frequency score ≤1 [2].
At baseline, 89% in the placebo group and 90% in the upadacitinib group suffered from abdominal pain (i.e. abdominal pain score >0). “This is strikingly high. I did not realise so many have abdominal pain at baseline because we never measure it,” Prof. D´Haens commented. At the end of the maintenance period at week 52, 46.1% achieved complete symptom resolution in the high-dose upadacitinib group compared with 37.2% in the low-dose upadacitinib group and 12.8% in the placebo group (P<0.001 for both comparisons; see Figure).
Figure: Percentage of patients with symptom resolution and induction response during maintenance treatment [2]
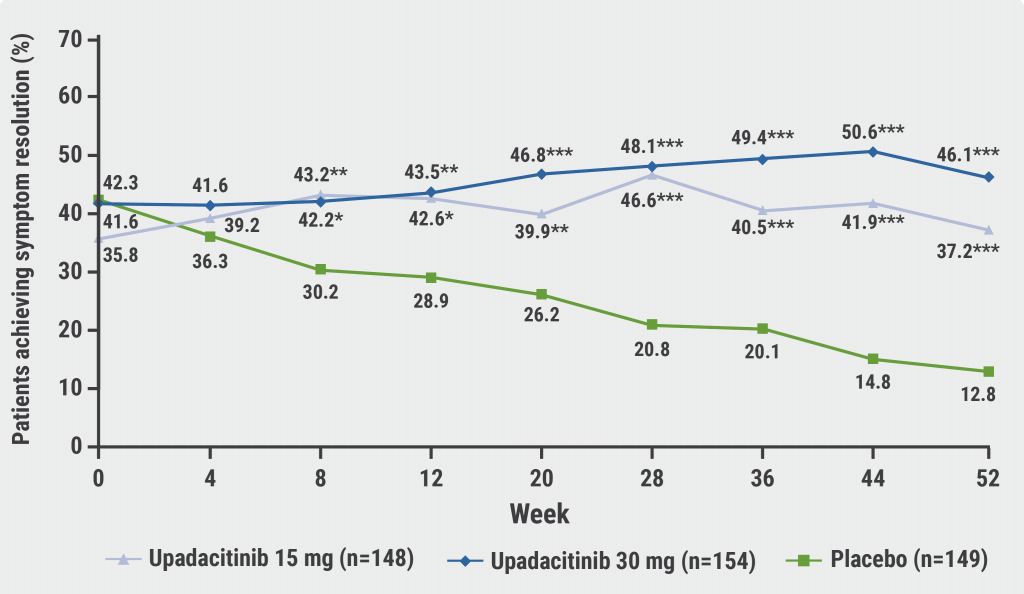
*P<0.05; **P<0.01, ***P<0.001 for upadacitinib versus placebo.
If a fatigue score was added, 36.4% of participants treated with 30 mg upadacitinib achieved both complete symptom resolution and fatigue normalisation compared with 12.1% of placebo at week 52 (P<0.001). Complete symptom resolution and normalisation of fatigue were sustained with both doses of upadacitinib maintenance treatment. Achieving this goal may contribute to the normalisation of disease-related quality-of-life in patients with UC, according to Prof. D´Haens.
- Feagan BG, et al. Upadacitinib improves endoscopic outcomes in patients with moderate to severely active Crohn´s Disease irrespective of previous failure to respond to biologics or conventional therapy. OP17, ECCO 2023, 1–4 March, Copenhagen, Denmark.
- D´Haens G, et al. The effects of upadacitinib on ulcerative colitis symptom resolution and fatigue normalisation in patients with moderately to severely active ulcerative colitis: Phase 3 U-ACHIEVE and U-ACCOMPLISH result. OP20, ECCO 2023, 1–4 March, Copenhagen, Denmark.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Solid results for long-term therapy of UC with filgotinib Next Article
Continued efficacy of long-term ozanimod as UC treatment »
« Solid results for long-term therapy of UC with filgotinib Next Article
Continued efficacy of long-term ozanimod as UC treatment »
Table of Contents: ECCO 2023
Featured articles
What Is New in Biologic Therapy?
Beneficial effect of early, post-operative vedolizumab on endoscopic recurrence in CD
Long-term data supports the established efficacy and safety of ustekinumab in UC
Anti-TNF withdrawal may be a safe option in stable IBD
Intensified drug therapy leads to better stricture morphology in CD
Small Molecules in IBD: State of the Art
Continued efficacy of long-term ozanimod as UC treatment
Upadacitinib successful in the management of both CD and UC
Solid results for long-term therapy of UC with filgotinib
Paediatric IBD: What You Need To Know
Perinatal period is crucial for the risk of developing CD
Early-life antibiotic exposure: a risk factor for paediatric-onset IBD
Paediatric patients with immune-mediated inflammatory disease harbour a heightened cancer risk
Risk Factors and Complications of IBD
Checking kidney function is important during the course of IBD
Diabetes therapy with GLP-1-based drugs does not elevate the risk of IBD
Surgical Approaches: New Developments
Long-term resection potentially better than anti-TNF treatment in CD
Early, post-operative complications in CD reduced by pre-operative enteral nutrition, irrespective of biologic exposure
Pearls of the Posters
Drop in overall IBD procedures during the pandemic
Proton pump inhibitors associated with worse outcomes in CD
Poor sleep in CD linked to low levels of vitamin D
Novel AI tool assessing mucosal inflammation achieves high correlation with histopathologists
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

