https://doi.org/10.55788/8ca6a63c
“For the first time, an endoscopy outcome is included in a long-term study,” emphasised Prof. Silvio Danese (Vita-Salute San Raffaele University, Italy). Prof. Danese presented the final clinical outcomes based on the full Mayo score, including the endoscopy subscore, of the UNIFI LTE (NCT02407236) study through 4 years of ustekinumab treatment [1]. The IL-12/23p40 monoclonal antibody ustekinumab has already been approved for the treatment of moderate-to-severe UC.
The maintenance study re-randomised 523 participants who responded to ustekinumab therapy 8 weeks after intravenous induction. In the maintenance study, the participants were treated with either 90 mg ustekinumab every 8 weeks or every 12 weeks. The participants who completed week 44 (n=284), entered a long-term extension until week 200. From week 56 onwards, dose adjustments were possible at any time based on the clinical judgement of the investigator. In the analysis, all patients with long-term extension were included who either had Mayo score data including endoscopy at week 200 or experienced treatment failure before week 200 (imputed as non-responder).
At week 200, 58% of the participants were in clinical remission (defined as a Mayo score ≤2 points and no individual subscore >1; see Figure). “Overall, there were very similar results for the 12-week and 8-week dose intervals,” Prof. Danese said. 80% of participants showed a clinical response (defined as a decrease in Mayo score of ≥30% and ≥3 points from the induction baseline with either a decrease in rectal bleeding subscore of ≥1 from the induction baseline or a rectal bleeding subscore of 0 or 1). In addition, 67.3% of participants achieved endoscopic improvement with an endoscopy subscore of 0 or 1, with no major differences according to the dose intervals. “Around 30% of patients need a shorter dose interval,” Prof. Danese commented on his experience.
Figure: Clinical and endoscopic outcomes after 4 years of ustekinumab treatment in the UNIFI LTE study [1]
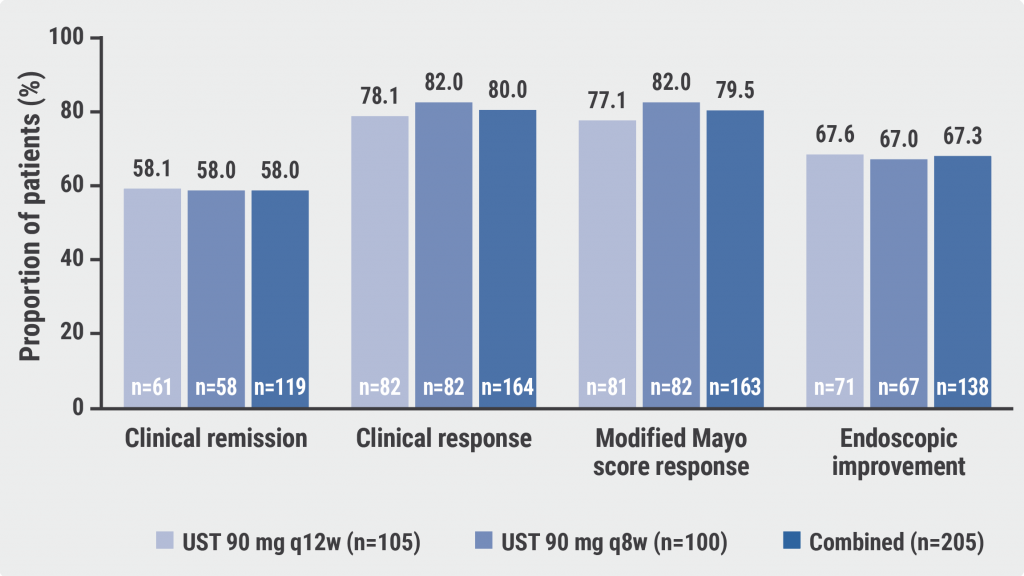
Q8w, every 8 weeks; q12w; every 12 weeks; UST, ustekinumab.
In conclusion, the UNIFI LTE study showed that approximately two-thirds of participants with moderate-to-severe UC treated with ustekinumab were in clinical remission, clinical response, and/or demonstrated endoscopic improvement after 4 years. With continued ustekinumab treatment in the long-term extension, clinical remission was generally maintained through week 200 among participants who were in clinical remission at maintenance baseline or after 1 year of ustekinumab maintenance treatment.
- Danese S, et al. Efficacy of ustekinumab for Ulcerative Colitis through 4 years: Final clinical and endoscopy outcomes from the UNIFI long-term extension. OP15, ECCO 2023, 1-4 March, Copenhagen, Denmark.
Posted on
Previous Article
« Anti-TNF withdrawal may be a safe option in stable IBD Next Article
Beneficial effect of early, post-operative vedolizumab on endoscopic recurrence in CD »
« Anti-TNF withdrawal may be a safe option in stable IBD Next Article
Beneficial effect of early, post-operative vedolizumab on endoscopic recurrence in CD »
Table of Contents: ECCO 2023
Featured articles
What Is New in Biologic Therapy?
Beneficial effect of early, post-operative vedolizumab on endoscopic recurrence in CD
Long-term data supports the established efficacy and safety of ustekinumab in UC
Anti-TNF withdrawal may be a safe option in stable IBD
Intensified drug therapy leads to better stricture morphology in CD
Small Molecules in IBD: State of the Art
Continued efficacy of long-term ozanimod as UC treatment
Upadacitinib successful in the management of both CD and UC
Solid results for long-term therapy of UC with filgotinib
Paediatric IBD: What You Need To Know
Perinatal period is crucial for the risk of developing CD
Early-life antibiotic exposure: a risk factor for paediatric-onset IBD
Paediatric patients with immune-mediated inflammatory disease harbour a heightened cancer risk
Risk Factors and Complications of IBD
Checking kidney function is important during the course of IBD
Diabetes therapy with GLP-1-based drugs does not elevate the risk of IBD
Surgical Approaches: New Developments
Long-term resection potentially better than anti-TNF treatment in CD
Early, post-operative complications in CD reduced by pre-operative enteral nutrition, irrespective of biologic exposure
Pearls of the Posters
Drop in overall IBD procedures during the pandemic
Proton pump inhibitors associated with worse outcomes in CD
Poor sleep in CD linked to low levels of vitamin D
Novel AI tool assessing mucosal inflammation achieves high correlation with histopathologists
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

