https://doi.org/10.55788/40772b88
A first-in-class, investigational, oral, IL-23 receptor antagonist peptide, provisionally named JNJ-77242113, was associated with improved efficacy outcomes compared with placebo in patients with moderate-to-severe plaque psoriasis, in a phase 2b study.
“There are currently no orally delivered therapeutics for psoriasis selectively targeting the IL-23 pathway,” said Dr Robert Bissonnette (Innovaderm Research, Canada) [1]. JNJ-77242113 is a first-in-class, oral, IL-23 antagonist peptide. “Because of the gastrointestinal stability and potency of this investigational agent, it is able to deliver systemic IL-23 pathway blockade via oral dosing,” added Dr Bissonnette.
The FRONTIER 1 trial (NCT05223868) randomised 255 patients with moderate-to-severe plaque psoriasis 1:1:1:1:1:1 to 1 of 5 doses of JNJ-77242113, ranging from 25 mg once daily to 100 mg twice daily, or a placebo. Dr Bissonnette and colleagues looked primarily at the proportion of participants achieving Psoriasis Area and Severity Index (PASI)75 after 16 weeks of treatment [2].
A significant dose-response was observed for PASI75 after 16 weeks of therapy: 9.3% of the participants on placebo reached PASI75 compared with 37.2% (P<0.01) and 78.6% (P<0.001) of the participants on the lowest dose and highest dose of JNJ-77242113, respectively (see Figure). Furthermore, 40.5% of the participants on the highest dose of JNJ-77242113 reached PASI100 compared with none on placebo.
Figure: Proportion of participants achieving PASI75 at week 16 [2]
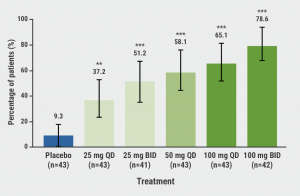
BID, twice daily; QD, once daily.
Dr Bissonnette added that patients responded quickly to the agent, with over 20% of the participants in the high-dose group achieving PASI75 at week 4. In terms of Investigator’s Global Assessment (IGA) response, 64.3% of the participants in the highest dose group achieved a score of 0 or 1, compared with 11.6% of the participants in the placebo. In addition, 45.2% of the participants in the high-dose group reached an IGA score of 0, whereas none of the participants on placebo reached this score.
No dose-related trends were observed regarding adverse events. The proportions of participants who experienced at least 1 adverse event were comparable for the 6 arms of the study. COVID-19 (10.8%) and nasopharyngitis (7.1%) were the most frequently reported adverse events.
In conclusion, JNJ-77242113 showed promising efficacy results in this phase 2b study and was well tolerated in a population of patients with moderate-to-severe plaque psoriasis.
- Fourie A, et al. Presented at ISID meeting; May 10-13, 2023; Tokyo, Japan. ID1109.
- Bissonnette R, et al. A phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Late-breaker Session 1, WCD 2023, 3–8 July, Singapore, Singapore.
Posted on
« Subcutaneous spesolimab for GPP flare prevention Next Article
POETYK PSO-1 and 2: Long-term efficacy results of deucravacitinib in plaque psoriasis »
Table of Contents: WCD 2023
Featured articles
Atopic Dermatitis
Rocatinlimab delivers efficacy and safety in atopic dermatitis
Head-to-head: paraffin- versus ceramide-based moisturiser for paediatric AD
Novel JAK1 inhibitor for patients with atopic dermatitis
Can lebrikizumab maintain response rates in atopic dermatitis?
Most patients with AD on dupilumab stick with this drug long-term
Psoriasis
Botulinum toxin A might provide efficacious treatment option for nail psoriasis
POETYK PSO-1 and 2: Long-term efficacy results of deucravacitinib in plaque psoriasis
Encouraging results for first oral IL-23 receptor antagonist in plaque psoriasis
Subcutaneous spesolimab for GPP flare prevention
Knocking out psoriasis with high-dose risankizumab?
Deucravacitinib versus other systemic therapies in Asian patients with psoriasis
Hair Disorders
Patients with AA report high long-term regrowth rates with baricitinib
Can ritlecitinib deliver long-term efficacy in alopecia areata?
TikTok videos on hair disorders lack reliability
Hidradenitis, Acne, and Rosacea
Spesolimab appears successful in hidradenitis suppurativa
Promising results for paroxetine in rosacea
Novel PPARγ modulator reduces acne manifestations
Microencapsulated benzoyl peroxide shifts skin microbiome in rosacea
Other Skin Conditions and Teledermatology
OLYMPIA 2: Positive results for nemolizumab in prurigo nodularis
PRFM or PRP therapy for trophic ulcers due to leprosy?
Large teledermatology project in a remote island in Eastern Indonesia
Can AI-driven teledermatology increase access to healthcare in rural African settings?
Oleogel-S10 shows long-term efficacy and safety in dystrophic epidermolysis bullosa
Picosecond alexandrite laser safe and effective in benign pigmentary disorders
Gentamicin improves symptoms in paediatric Nagashima-type palmoplantar keratosis
Related Articles
OLYMPIA 2: Positive results for nemolizumab in prurigo nodularis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

