A pooled analysis from 2 phase 3 trials found that secukinumab treatment entailed a numerically greater pain reduction than placebo in patients with hidradenitis suppurativa (HS). More than 65% of patients with moderate/severe pain at baseline, decreased to mild/no pain after 52 weeks.
Dr John Ingram (Cardiff University, UK) presented the post-hoc analysis of the phase 3 SUNSHINE (NCT03713619) and SUNRISE (NCT03713632) trials that focused on pain ameliorations with secukinumab for HS treatment [1]. Previously, the 2 identical trials had found the superiority of the IL-17A inhibitor over placebo for a bi-weekly dosing (Q2W) with 300 mg at week 16. Within the 2 trials, pain was measured by the Patient’s Global Assessment (PGA) of skin pain on a numeric rating scale (NRS). The current analysis evaluated changes in skin pain at its worst in different categories of intensity: no pain: NRS 0, mild pain: NRS >0–≤6, moderate pain: NRS >6–≤8, and severe pain: NRS >8.
SUNSHINE and SUNRISE included over 1,000 participants with a mean age of 36.2 years, 56.3% were women, and the mean baseline pain was NRS 5.2. At week 16, secukinumab numerically decreased pain in comparison to placebo: Q2W NRS -1.4, Q4W NRS -1.1 versus placebo NRS -0.5. These pain ameliorations were continued to week 52, with a numerically greater decrease on secukinumab Q2W (NRS -1.8) than Q4W (NRS -1.5).
Looking at reductions after 1 year by pain category, 8.3% of the participants on Q2W secukinumab with moderate pain and 8.6% with severe pain at baseline achieved a status of no pain. Also, 63.3% and 45.7% of these groups experienced a reduction to mild pain, respectively. After dichotomising into NRS >6 and NRS≤6, 65.3% (Q2W) and 70.1% (Q4W) of those with moderate/severe pain at baseline had mild/no pain after 52 weeks.
In their conclusion, the authors added that secukinumab treatment also entailed a decreased use of pain medication over time and an improvement in quality of life.
- Ingram JR, et al. Secukinumab provides sustained improvements in pain in patients with moderate to severe hidradenitis suppurativa: A post-hoc analysis of the SUNSHINE and SUNRISE phase 3 trials. P0045, EADV Congress 2023, 11–14 October, Berlin, Germany.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Nemolizumab shows high success rates in prurigo nodularis Next Article
Botanical drug solution improves hair regrowth in children and adolescents with AA »
« Nemolizumab shows high success rates in prurigo nodularis Next Article
Botanical drug solution improves hair regrowth in children and adolescents with AA »
Table of Contents: EADV 2023
Featured articles
Tapinarof on course to become a new topical treatment in AD
AD and Eczema in 2023
Tapinarof on course to become a new topical treatment in AD
Upadacitinib provides sustained skin clearance in adolescents and adults with AD
Sustained deep clinical and itch responses with novel IL-13 inhibitor
IL-13 inhibitor shows potential in atopic dermatitis
Encouraging results for amlitelimab in atopic dermatitis
Chronic hand eczema: patients share similar molecular signatures regardless of AD status
Severe hand eczema: dupilumab could be a future treatment
Psoriasis News
Dual IL-17 blockade yields efficacy on joints and skin
High-dose subcutaneous spesolimab prevents GPP flares up to week 48
Drug survival of guselkumab and risankizumab seems superior to other biologics
IL-23 blockers may lower the risk of developing inflammatory and psoriatic arthritis
First-in-class oral IL-23 inhibitor safe and effective for moderate-to-severe plaque psoriasis
Hidradenitis Suppurativa: End of the Diagnostic and Therapeutic Draught
Skin tape stripping allows a novel precision medicine approach in HS
Nanobodies: A novel way to treat HS
Anti-IL17 blockade leads to maintained pain reduction in patients with HS
Vitiligo: Novel Treatment Options
JAK1 inhibition: a promising forthcoming treatment option in vitiligo
Vitiligo: Continuation of topical ruxolitinib successful in many initial non-responders
Alopecia Areata: Novel Developments
JAK3/TEC inhibition achieves clinically meaningful responses in AA
Alopecia areata: remarkable regrowth rates with deuruxolitinib
Botanical drug solution improves hair regrowth in children and adolescents with AA
What’s New in Other Disease Entities
Nemolizumab shows high success rates in prurigo nodularis
Remibrutinib reduces itch, sleep problems, and activity impairment in patients with CSU
Innovative wound gel reduces frequency of painful dressing changes in epidermolysis bullosa
Best of the Posters
Women with psoriasis face increased adverse effects with systemic therapy
Improved AI tool shows high sensitivity rates in skin cancer detection
Dermoscopy training combined with AI significantly improves skin cancer detection
Related Articles
May 10, 2023
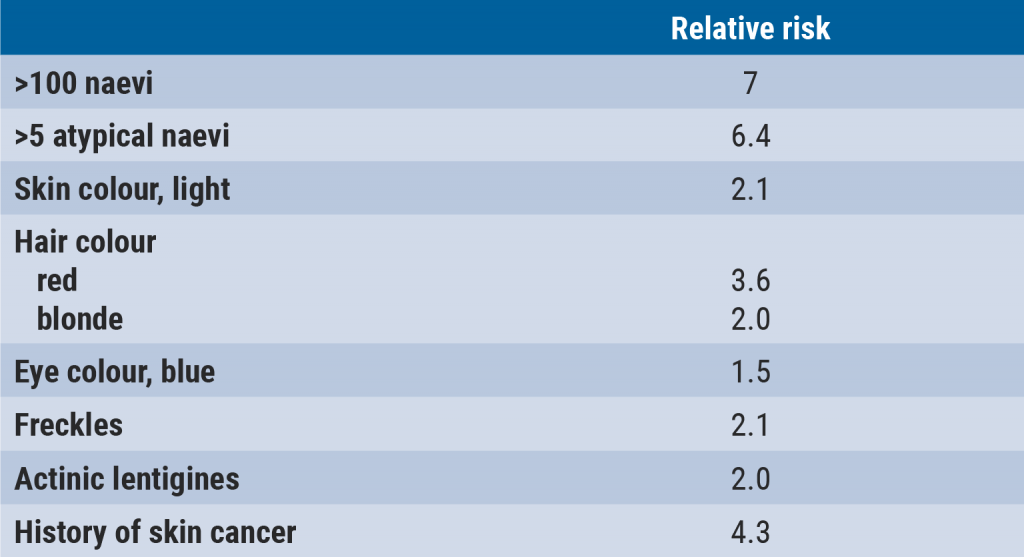
Melanoma: Surveillance and follow-up
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

