https://doi.org/10.55788/b3195d65
Seborrheic dermatitis has a prevalence of around 5% worldwide [1,2]. “It’s a disease that is very common, yet in my opinion undertreated,” Prof. Andrew Blauvelt (Oregon Medical Research Center, OR, USA) stated [1]. Current therapy options include topical steroids and anti-fungals that may have some restrictions regarding side effects or difficulties in use in some body regions, especially the face and hair bearing areas.
Due to positive results in phase 2, a 0.3% foam preparation of the PDE4 inhibitor roflumilast was evaluated in the phase 3 STRATUM study (NCT04973228) on treatment for seborrheic dermatitis. After a 2:1 randomisation, participants received either the active drug foam once daily or a vehicle over 8 weeks. Recruited were 457 participants, among them 7% was between 9–17 years, as the disease already may occur in pubescents. The average age was 42, and there was an equal distribution of men and women.
All participants had an Investigator´s Global Assessment (IGA) ≥3, equalling at least moderate disease and an affected body surface area ≤20%. In practice, over 90% had an IGA of 3 at baseline and on average around 3% of the body surface was affected. Success, in terms of the primary endpoint, meant achieving an IGA of 0/1 corresponding to clear or almost clear skin. The most common body regions involved were scalp in around 90%, face in over 60%, and ears in more or less 50% of the trial population.
Early onset of action
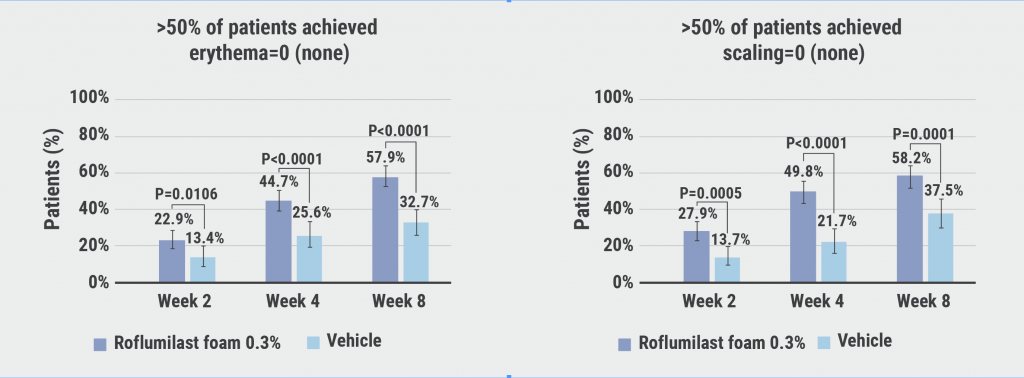
Statistical difference in favour of roflumilast already started at week 2. At week 8, 80.1% of participants attained success with an IGA of 0/1 on roflumilast in comparison with 59.2% on placebo (P<0.0001). Prof. Blauvelt attributed the high placebo rate to the possibility of a beneficial effect through the once daily moisturisation that the vehicle provided. Over half of the participants were completely clear of disease after 8 weeks (50.7%; P<0.0001). Furthermore, 57.9% (P<0.0001) and 58.2% (P<0.0001) achieved scores of 0 for erythema and scaling at week 8 (see Figure) and 63.6% (P=0.0002) experienced a 4-point reduction on the worst-itch numeric rating scale.
Figure: Efficacy and safety of roflumilast foam 0.3% in seborrheic dermatitis [1]
The foams with roflumilast and the vehicle were both well tolerated throughout the trial with 97.8% and 94.8% of participants reporting no or only mildly discomforting sensation at week 8. The safety profile did not raise concerns: any treatment-emergent events appeared in 23% on roflumilast versus 21.6% on placebo.
“Many patients responded in this trial, so much so that I called it the “happy trial”. Every time I saw patients in this trial, they seemed to be happy,” Prof. Blauvelt remarked in view of the results.
- Blauvelt A. Efficacy and safety of roflumilast foam 0.3% in patients with seborrheic dermatitis in a phase 3 trial. D2T01.3F, EADV Congress 2022, Milan, Italy, 7‒10 September
- Dessinioti C, et al. Clin Dermatol. 2013;31(4):343‒51.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Letter from the Editor Next Article
Does 8 weeks of emollients use prevent AD in high-risk infants? »
« Letter from the Editor Next Article
Does 8 weeks of emollients use prevent AD in high-risk infants? »
Table of Contents: EADV 2022
Featured articles
Letter from the Editor
Psoriasis and Psoriatic Arthritis: What You Need to Know
Novel oral psoriasis drug maintains efficacy over 2 years
A3 adenosine receptor agonist showed modest efficacy but excellent tolerability
Selective IL-23 inhibitor achieves long-term disease control in many patients with active PsA
AI machine learning algorithm useful in early detection of PsA
Novel Developments in Sun Protection
Myths regarding “health benefit” of suntan prevail in majority of population
Fern extract reverses severe actinic keratosis lesions
Vitiligo in 2022
Enhancing re-pigmentation rates with topical ruxolitinib in all body areas
Markedly lower skin cancer risk in vitiligo patients
Pruritus Treatment: Novel Agents Entering the Arena
Dupilumab leads to clinically relevant improvements in signs and symptoms of prurigo nodularis
Nalbuphine: aspiring to become another treatment for prurigo nodularis?
Notalgia paresthetica: may κ-opioid receptor agonists be a long-awaited effective therapy?
Pharmacotherapy in Hidradenitis Suppurativa: New Opportunities
High potential for secukinumab as next biologic treatment for HS
Hidradenitis suppurativa: TYK2/JAK1 inhibitor shows promise
Best of the Posters
High rate of non- or partial responders jeopardises therapeutic success in HS
Genital psoriasis: high prevalence, often underdiagnosed
Decreased overall survival in melanoma patients with low vitamin D
News in Atopic and Seborrheic Dermatitis
Baricitinib possible therapeutic option for children with AD
Amlitelimab therapy leads to sustained decrease of IL-22 in AD patients
IL-13 inhibition with lebrikizumab shows high maintenance rates in AD
Does 8 weeks of emollients use prevent AD in high-risk infants?
Roflumilast foam led to high response rates in seborrheic dermatitis
What Is Hot in Hair Disorders?
Long-term improvement in alopecia areata with ritlecitinib therapy
Topical gel plus finasteride beneficial for patients with androgenetic alopecia
Deuruxolitinib achieves hair regrowth, even in patients with severe alopecia areata
Related Articles
November 5, 2022
Long-term improvement in alopecia areata with ritlecitinib therapy
November 5, 2022
Does 8 weeks of emollients use prevent AD in high-risk infants?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

