https://doi.org/10.55788/4b1b82b5
Baricitinib, a JAK inhibitor, has been approved in several countries for the treatment of AD in adult patients and its efficacy has been proven in different studies. “Therefore, it is mandatory to know if this drug is also effective in children and adolescents,” stated Dr Antonio Torrelo Fernández (Hospital Infantil Universitario Niño Jesús, Spain) [1]. The phase 3, randomised, placebo-controlled BREEZE-AD-PEDS trial (NCT03952559) randomised 483 patients aged 2 to 18 years to receive baricitinib either at a low, medium, or high dose, or placebo. Dose regimens were adjusted for participants aged <10 years or 10 to 18 years, with daily 0.5 mg/1 mg, 1 mg/2 mg, or 2 mg/4 mg baricitinib. A validated Investigator’s Global Assessment (vIGA) score of clear or almost clear skin (0/1) with at least 2 points improvement from baseline marked the primary endpoint at week 16. To be included, patients needed to have moderate-to-severe AD with a history of inadequate response or intolerance of topical treatment plus a disease duration of at least 6 or 12 months, depending on age.
Dr Torrelo Fernández underlined that the 4 study arms were similar in age, gender, race, and geographical region. The mean age was around 12 years and the mean duration of AD was at least 9 years. Participants with vIGA 4 made up between 37.5% and 39.3% of the participants, all other participants had vIGA 3.
In the high-dose baricitinib group, 41.7% reached the primary endpoint at week 16, significantly more than the placebo group (16.4%; P<0.001; see Figure). For the secondary endpoints Eczema Area and Severity Index (EASI)-75 and EASI-90, significant difference was similarly present in the high-dose arm, with 52.6% and 30% responders, respectively (P<0.01). Dr Torrelo Fernández further highlighted that 35.5% of the patients on 2 mg/4 mg of baricitinib had a ≥4-point reduction in the itch numeric rating scale (P<0.05). Dr Torrelo Fernández stated that the vIGA results were consistent in subgroups according to age <10 or ≥10 years and across 3 different weight groups. Of note, there was a very high study adherence with 96.7% of participants completing week 16.
Figure: Percentage responders with vIGA-AD(0/1) with ≥2 points improvement [1]
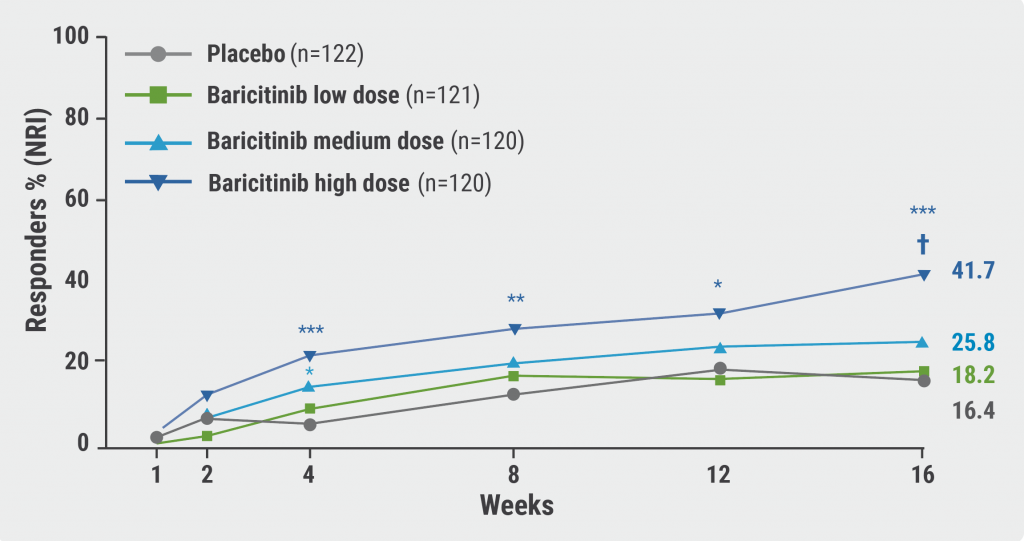
*, P<0.05; **, P<0.01; ***, P<0.001; †, statistically significant with multiplicity adjustment; NRI, non-responder imputation.
Any treatment-emergent adverse event occurred in 50% of the low-dose and placebo arm, 52.5% in the medium-dose arm, and 50.8% in the high-dose arm. “The severity was low, mild, or moderate in most of the cases and severe adverse events were reported very seldomly,” Dr Torrelo Fernández stated. The most common were headache, acne, abdominal pain, and nasopharyngitis. “But you can see again that these adverse events were similarly seen in all 4 groups,” Dr Torrelo Fernández stressed.
In summary, baricitinib was assessed as a potential therapeutic option with a favourable benefit-risk profile for children and adolescents between 2 and 18 years, who have moderate-to-severe AD and who are candidates for systemic therapy.
- Torello F. Efficacy and safety of baricitinib in a phase 3, randomised, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe atopic dermatitis. D3T01.1E, EADV Congress 2022, Milan, Italy, 7–10 September.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Amlitelimab therapy leads to sustained decrease of IL-22 in AD patients Next Article
Deuruxolitinib achieves hair regrowth, even in patients with severe alopecia areata »
« Amlitelimab therapy leads to sustained decrease of IL-22 in AD patients Next Article
Deuruxolitinib achieves hair regrowth, even in patients with severe alopecia areata »
Table of Contents: EADV 2022
Featured articles
Letter from the Editor
Psoriasis and Psoriatic Arthritis: What You Need to Know
Novel oral psoriasis drug maintains efficacy over 2 years
A3 adenosine receptor agonist showed modest efficacy but excellent tolerability
Selective IL-23 inhibitor achieves long-term disease control in many patients with active PsA
AI machine learning algorithm useful in early detection of PsA
Novel Developments in Sun Protection
Myths regarding “health benefit” of suntan prevail in majority of population
Fern extract reverses severe actinic keratosis lesions
Vitiligo in 2022
Enhancing re-pigmentation rates with topical ruxolitinib in all body areas
Markedly lower skin cancer risk in vitiligo patients
Pruritus Treatment: Novel Agents Entering the Arena
Dupilumab leads to clinically relevant improvements in signs and symptoms of prurigo nodularis
Nalbuphine: aspiring to become another treatment for prurigo nodularis?
Notalgia paresthetica: may κ-opioid receptor agonists be a long-awaited effective therapy?
Pharmacotherapy in Hidradenitis Suppurativa: New Opportunities
High potential for secukinumab as next biologic treatment for HS
Hidradenitis suppurativa: TYK2/JAK1 inhibitor shows promise
Best of the Posters
High rate of non- or partial responders jeopardises therapeutic success in HS
Genital psoriasis: high prevalence, often underdiagnosed
Decreased overall survival in melanoma patients with low vitamin D
News in Atopic and Seborrheic Dermatitis
Baricitinib possible therapeutic option for children with AD
Amlitelimab therapy leads to sustained decrease of IL-22 in AD patients
IL-13 inhibition with lebrikizumab shows high maintenance rates in AD
Does 8 weeks of emollients use prevent AD in high-risk infants?
Roflumilast foam led to high response rates in seborrheic dermatitis
What Is Hot in Hair Disorders?
Long-term improvement in alopecia areata with ritlecitinib therapy
Topical gel plus finasteride beneficial for patients with androgenetic alopecia
Deuruxolitinib achieves hair regrowth, even in patients with severe alopecia areata
Related Articles
November 5, 2022
Long-term improvement in alopecia areata with ritlecitinib therapy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

