https://doi.org/10.55788/6ec2f53f
Similar to baricitinib, which is already approved for AA in Europe and Japan, deuruxolitinib also targets JAK1 and JAK2. In the multinational, phase 3 THRIVE-AA1 trial (NCT04518995), adult patients with severe AA were included to assess the efficacy and safety of deuruxolitinib with a study duration of up to 32 weeks [1]. Participants were treated with either 8 mg or 12 mg deuruxolitinib twice daily or placebo and had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT). The primary efficacy endpoint was the percentage of participants achieving a SALT score of ≤20 at week 24. “We also looked at patient satisfaction,” Prof. Brett King (Yale University School of Medicine, CT, USA) said. The percentage of responders, defined as “satisfied” or “very satisfied” on the Hair Satisfaction Patient-Reported Outcome (SPRO) scale at week 24 was assessed as a key secondary outcome.
At baseline, participants had a mean SALT score of 88. At week 24, participants treated with both doses of deuruxolitinib achieved the primary endpoint: 42% in the 12 mg dose group and 30% in the 8 mg dose group, compared with 1% in the placebo group achieved a SALT score ≤20 (P<0.0001 vs placebo; see Figure). “In AA, this is truly transformative, we see a very low placebo rate. In severe disease, the chance of spontaneous remission is close to 0,” Prof. King said. In the low-dose and high-dose groups, 21% and 35% respectively, achieved an even more stringent SALT score of ≤10 (P<0.0001 vs placebo).
Figure: Proportion of patients achieving SALT score ≤20 at week 24 [1]
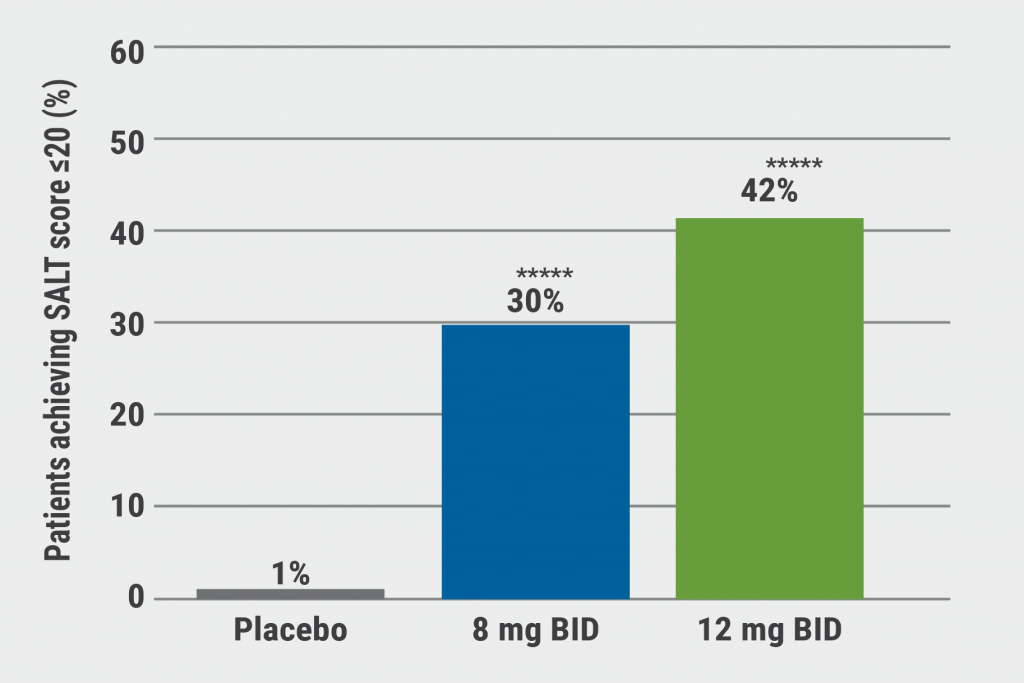
SALT, Severity of Alopecia Tool; BID, twice daily; *****, P<0.0001 vs placebo.
Significant changes in SALT score were seen as early as week 4. The agent also led to a significant improvement in eyebrow regrowth. “We wanted to know whether our impression aligns with what patients feel. Indeed, we see a high degree of patient satisfaction with scalp hair,” Prof. King said, with 42% responders in the low-dose group and 52% responders in the high-dose group.
Overall, the agent was generally well tolerated and more than 97% of participants will roll over into the open-label, long-term, extension study. According to Prof. King, this data is highly encouraging and supports the potential of deuruxolitinib to regrow hair on the scalp, eyebrows, and eyelashes in patients with AA.
- King B. Top-line results from THRIVE-AA1: A clinical trial of CTP-543 (Deuruxolitinib), an oral JAK inhibitor, in adult patients with moderate to severe alopecia areata. D3T01.1L, EADV Congress 2022, Milan, Italy, 7–10 September.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Baricitinib possible therapeutic option for children with AD Next Article
Topical gel plus finasteride beneficial for patients with androgenetic alopecia »
« Baricitinib possible therapeutic option for children with AD Next Article
Topical gel plus finasteride beneficial for patients with androgenetic alopecia »
Table of Contents: EADV 2022
Featured articles
Letter from the Editor
Psoriasis and Psoriatic Arthritis: What You Need to Know
Novel oral psoriasis drug maintains efficacy over 2 years
A3 adenosine receptor agonist showed modest efficacy but excellent tolerability
Selective IL-23 inhibitor achieves long-term disease control in many patients with active PsA
AI machine learning algorithm useful in early detection of PsA
Novel Developments in Sun Protection
Myths regarding “health benefit” of suntan prevail in majority of population
Fern extract reverses severe actinic keratosis lesions
Vitiligo in 2022
Enhancing re-pigmentation rates with topical ruxolitinib in all body areas
Markedly lower skin cancer risk in vitiligo patients
Pruritus Treatment: Novel Agents Entering the Arena
Dupilumab leads to clinically relevant improvements in signs and symptoms of prurigo nodularis
Nalbuphine: aspiring to become another treatment for prurigo nodularis?
Notalgia paresthetica: may κ-opioid receptor agonists be a long-awaited effective therapy?
Pharmacotherapy in Hidradenitis Suppurativa: New Opportunities
High potential for secukinumab as next biologic treatment for HS
Hidradenitis suppurativa: TYK2/JAK1 inhibitor shows promise
Best of the Posters
High rate of non- or partial responders jeopardises therapeutic success in HS
Genital psoriasis: high prevalence, often underdiagnosed
Decreased overall survival in melanoma patients with low vitamin D
News in Atopic and Seborrheic Dermatitis
Baricitinib possible therapeutic option for children with AD
Amlitelimab therapy leads to sustained decrease of IL-22 in AD patients
IL-13 inhibition with lebrikizumab shows high maintenance rates in AD
Does 8 weeks of emollients use prevent AD in high-risk infants?
Roflumilast foam led to high response rates in seborrheic dermatitis
What Is Hot in Hair Disorders?
Long-term improvement in alopecia areata with ritlecitinib therapy
Topical gel plus finasteride beneficial for patients with androgenetic alopecia
Deuruxolitinib achieves hair regrowth, even in patients with severe alopecia areata
Related Articles
November 5, 2022
Hidradenitis suppurativa: TYK2/JAK1 inhibitor shows promise

September 27, 2022
EADV 2022 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

