https://doi.org/10.55788/d5112dfd
Previous research has shown that disruption of IFN-γ signalling by inhibiting the JAK/STAT pathway is an attractive therapeutic target for vitiligo [1,2]. Thus, the objective of the current phase 2 dose-ranging study (NCT04927975) was to evaluate the efficacy and safety of the JAK1 inhibitor upadacitinib for the treatment of adults with non-segmental vitiligo, a disease with only limited treatment options [3]. Prof. Thierry Passeron (Nice University Hospital, France) presented the results.
The 184 participants were randomised to placebo or upadacitinib at either 6 mg, 11 mg, or 22 mg once daily for 24 weeks. The primary endpoint was the percentage change from baseline in the Facial Vitiligo Area Scoring Index (F-VASI) at week 24. After the double-blind period at week 24, participants on placebo were switched to either 11 mg or 22 mg upadacitinib and continuously treated until week 52.
The study met its primary endpoint with a significantly greater percentage change with the 2 highest upadacitinib doses (i.e. 11 mg and 22 mg). F-VASI was reduced by -34% in the 22 mg group (P<0.05 vs placebo) and -35.6% in the 11 mg group (P<0.01 vs placebo), compared with -14.4% in the placebo group (see Figure). Significant reductions were also noted for the change in total VASI (T-VASI).
Figure: Primary endpoint of percentage change from baseline in F-VASI at week 24 (MMRM) [3]
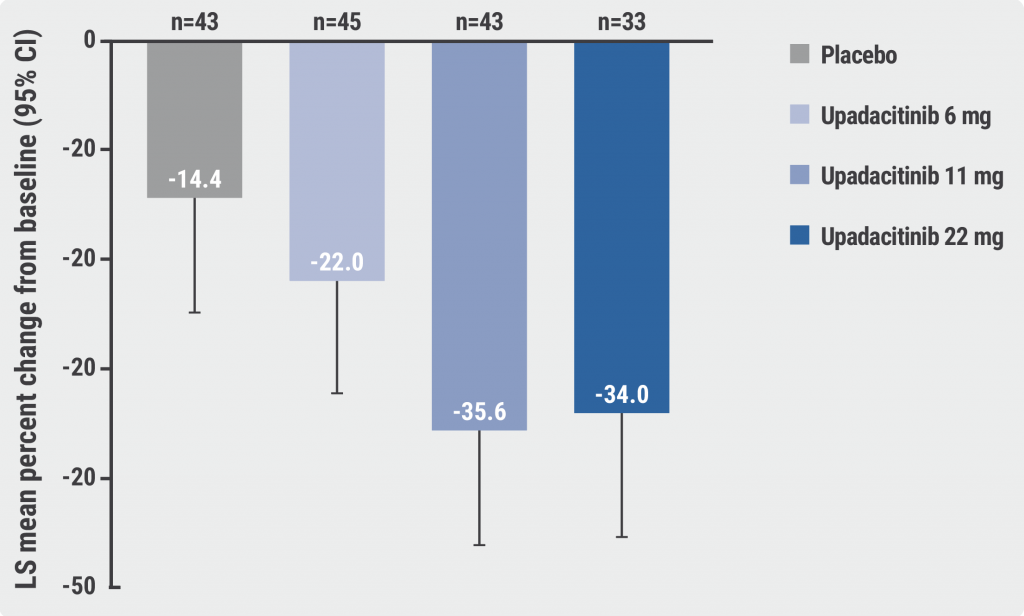
BL, baseline; CI, confidence interval; F-VASI, Facial Vitiligo Area Scoring Index; LS, least squares; MMRM, mixed-effects model for repeated measures; PBO, placebo; UPA, upadacitinib.
Moreover, participants treated with upadacitinib experienced continued improvement in F-VASI through week 52. At this time, participants achieved an approximately 60% to 65% reduction in F-VASI with 11 mg and 22 mg upadacitinib, respectively. Similar improvements in F-VASI were observed in participants who switched at week 24 from placebo to upadacitinib 11 mg or 22 mg. T-VASI values also improved up to week 52.
The safety assessment disclosed similar rates of treatment-emergent adverse events between upadacitinib and placebo groups with the most frequently reported treatment-emergent adverse events being a COVID-19 infection, acne, headache, and nasopharyngitis.
Relevant reading:
- Qi F, et al. Front Immunol 2021;12:790125.
- Boniface K, et al. Front Immunol 2021;12:613056.
- Passeron T. Efficacy and safety after 52 weeks of once-daily upadacitinib in adults with extensive non-segmental vitiligo (NSV): final results from a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. LB1, 2024 AAD Annual Meeting, 08–12 March, San Diego, USA.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« JAK1 inhibitor meets primary endpoint in prurigo nodularis Next Article
Next Post »
« JAK1 inhibitor meets primary endpoint in prurigo nodularis Next Article
Next Post »
Table of Contents: AAD 2024
Featured articles
New Developments in Dermatology
Upadacitinib: A novel treatment option for vitiligo
JAK1 inhibitor meets primary endpoint in prurigo nodularis
Botanical drug solution leads to sustained hair regrowth in paediatric alopecia
SGLT2 inhibition: A possible mode-of-action for inflammatory skin diseases?
Promising first results of novel topical treatment for congenital ichthyosis
Ritlecitinib also effective in patients with total hair loss
Atopic Dermatitis and Eczema in 2024
Amlitelimab leads to a high response 28 weeks after treatment discontinuation
Delgocitinib cream: A promising treatment option for chronic hand eczema
The Latest in Psoriasis
Robust long-term efficacy of bimekizumab in psoriasis
Benefit and safety of TYK2 inhibitor ESK-001 for psoriasis in phase 2
Durable skin clearance by IL-23 blockers due to reduction of resident memory T cells
Hidradenitis Suppurativa: New Treatment Possibilities
HS: Targeting IL-1 pathway potential option after anti-TNF failure
BTK signalling as a novel target in hidradenitis suppurativa treatment
Topical ruxolitinib shows promise in milder stages of hidradenitis suppurativa
Best of the Posters
Children with atopic dermatitis may be smaller and heavier than healthy children
JAK inhibitors have similar incidence rates of long-term adverse events as traditional immunomodulators
Baricitinib maintains regrowth of hair, eyebrows, and eyelashes over 3 years
GUIDE demonstrates: Hit hard and early in psoriasis
Hidradenitis suppurativa treatment with secukinumab linked to low immunogenicity
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

