https://doi.org/10.55788/e3a4bb5d
A remaining unmet need in AA is an approved drug with a favourable benefit/risk profile for children and adolescents [1]. Recently, the randomised, double-blind, multicentre, phase 2/3 RAAINBOW trial (NCT03240627) assessed the efficacy and safety of the botanical drug solution coacillium (22.3%). RAAINBOW enrolled 62 participants aged 2–18 years with a Severity of Alopecia Tool (SALT) score of 25–50 (i.e. moderate AA) and 50–95 (i.e. severe AA). The SALT score is a weighted sum of the percentage of hair loss in the 4 quadrants of the scalp, ranging from 0 (i.e. no hair loss) to 100 (i.e. complete hair loss).
The solution met the primary endpoint with a mean change in SALT score of +22.9%, versus -8.0% in the placebo group (P<0.0001), and 26% even achieved at least a 40% relative reduction in SALT score [1]. Prof. Bianca Piraccini (University of Bologna, Italy) presented further results of the RAAINBOW study [2].
After coacillium discontinuation, SALT continued to improve from up to week 48, 24 weeks after the last application of the solution (see Figure). Of the participants treated with the botanical solution, 82% experienced hair growth after treatment discontinuation. Efficacy was positively correlated with improved quality-of-life, assessed in 2 different quality-of-life questionnaires.
Figure: Continued improvement in SALT scores after coacillium discontinuation [2]
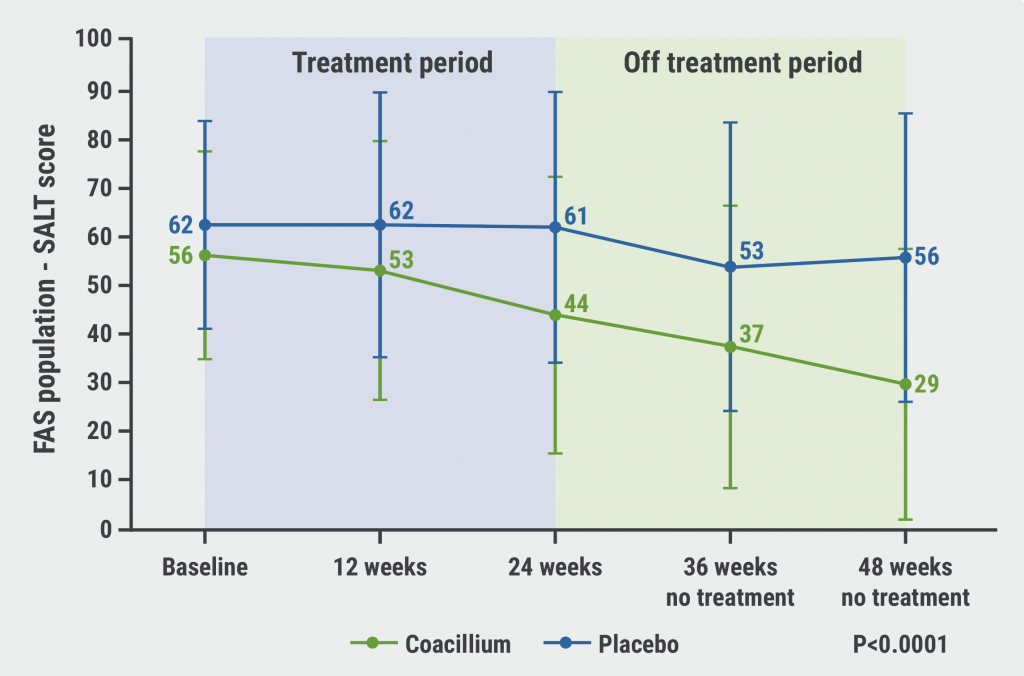
FAS, full analysis set; SALT, Severity of Alopecia Tool.
At week 48, almost half (46.7%) of the participants in the intervention arm achieved SALT scores ≤20 (compared with 9.1% in the placebo group; P=0.0031), a third of participants even gained a SALT score ≤10 (compared with 0% in the placebo group; P=0.0065). The average relative SALT change for responders to coacillium treatment was 41%.
The solution was generally well tolerated. As Prof. Piraccini emphasised in her conclusion, coacillium is the first drug to show sustained remission off-treatment in alopecia areata. The solution is rapidly absorbed by the hair follicle and is easy to apply.
Relevant reading:
- Blume-Peytavi U, et al. 1L, EADV Congress 2023, 11–14 October, Berlin, Germany.
- Piraccini BM. Efficacy and safety of coacillium in children and adolescents with moderate to severe alopecia areata: a randomised, double-blind, placebo-controlled, international, phase 2-3 trial. LB1, 2024 AAD Annual Meeting, 8–12 March, San Diego, USA.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« SGLT2 inhibition: A possible mode-of-action for inflammatory skin diseases? Next Article
JAK1 inhibitor meets primary endpoint in prurigo nodularis »
« SGLT2 inhibition: A possible mode-of-action for inflammatory skin diseases? Next Article
JAK1 inhibitor meets primary endpoint in prurigo nodularis »
Table of Contents: AAD 2024
Featured articles
New Developments in Dermatology
Upadacitinib: A novel treatment option for vitiligo
JAK1 inhibitor meets primary endpoint in prurigo nodularis
Botanical drug solution leads to sustained hair regrowth in paediatric alopecia
SGLT2 inhibition: A possible mode-of-action for inflammatory skin diseases?
Promising first results of novel topical treatment for congenital ichthyosis
Ritlecitinib also effective in patients with total hair loss
Atopic Dermatitis and Eczema in 2024
Amlitelimab leads to a high response 28 weeks after treatment discontinuation
Delgocitinib cream: A promising treatment option for chronic hand eczema
The Latest in Psoriasis
Robust long-term efficacy of bimekizumab in psoriasis
Benefit and safety of TYK2 inhibitor ESK-001 for psoriasis in phase 2
Durable skin clearance by IL-23 blockers due to reduction of resident memory T cells
Hidradenitis Suppurativa: New Treatment Possibilities
HS: Targeting IL-1 pathway potential option after anti-TNF failure
BTK signalling as a novel target in hidradenitis suppurativa treatment
Topical ruxolitinib shows promise in milder stages of hidradenitis suppurativa
Best of the Posters
Children with atopic dermatitis may be smaller and heavier than healthy children
JAK inhibitors have similar incidence rates of long-term adverse events as traditional immunomodulators
Baricitinib maintains regrowth of hair, eyebrows, and eyelashes over 3 years
GUIDE demonstrates: Hit hard and early in psoriasis
Hidradenitis suppurativa treatment with secukinumab linked to low immunogenicity
Related Articles
August 26, 2019
Various guidelines with much overlap
November 28, 2023
Skin tape stripping allows a novel precision medicine approach in HS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

