Dr Douglas Johns (Merck & Co., NJ, USA) mentioned that many patients with hypercholesterolaemia do not reach their LDL cholesterol treatment goals [1]. Although injectable PCSK9 inhibitors demonstrated LDL cholesterol reductions of 50–60%, these therapies are often administered as a last resort only. An oral PCSK9 inhibitor could remove the barriers associated with injectable treatments.
A first randomised, double-blind, placebo-controlled, in-human trial assessed the safety and tolerability of single doses of MK-0616 ranging from 10 mg to 300 mg in 60 male participants (aged 18–50 years). MK-0616 was generally safe and well tolerated in this population. No serious adverse events (AEs) were reported and only 1 treatment-related discontinuation was observed, a case of maculopapular rash. Drug-related AEs were mostly abdominal discomfort, diarrhoea, dyspepsia, and headache. Free PCSK9 was reduced by more than 80%, regardless of the administered dose of MK-0616. This effect lasted for approximately 24 hours. Free PCSK9 levels returned to baseline levels in 96 hours. In addition, the authors observed that a permeation enhancer (i.e. sodium caprate) improved the absorption of MK-0616 and noted a negative pre-dose food effect.
A second double-blind, placebo-controlled, phase 1 trial evaluated the LDL-cholesterol lowering capacities of MK-0616 in 40 men and women (aged 18–65 years) treated with statin therapy. Patients were randomised 3:1 to 1 of 3 dosing regimens of MK-0616 or placebo (see Figure). The 14-day result displayed no serious AEs, deaths, or discontinuations. Treatment-related AEs were similar to the reported AEs in the first trial, demonstrating a favourable safety profile of the agent. LDL cholesterol was reduced by a maximum of 65% in all experimental conditions, except for the pre-dose food condition (see Figure). This result suggests that low-dose MK-0616 plus low-dose sodium caprate can achieve a major reduction in LDL cholesterol. Larger clinical trials need to confirm the safety and efficacy of MK-0616 in a diverse population.
Figure: MK-0616 dosing and LDL-cholesterol change from baseline [1]
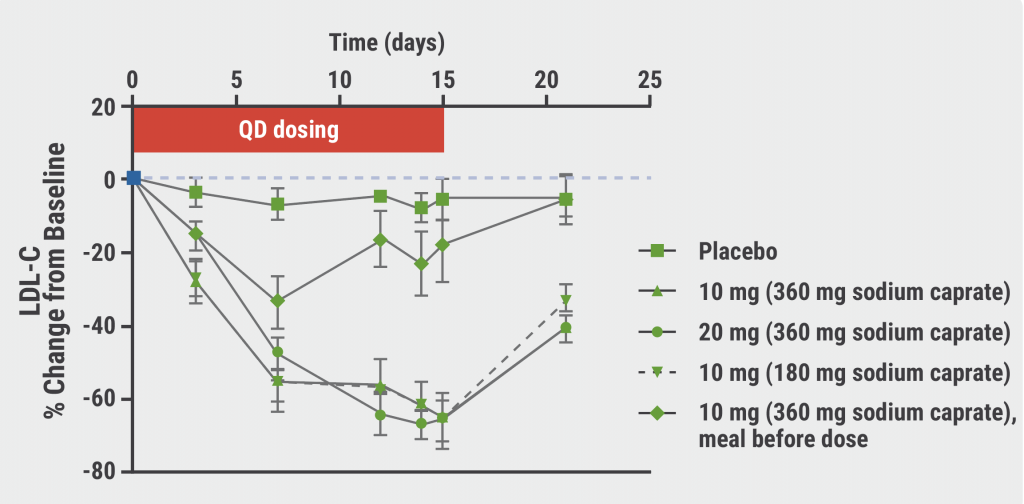
LDL-C, low-density lipoprotein cholesterol; PBO, placebo.
- Johns DG, et al. The Clinical Safety, Pharmacokinetics, and LDL-Cholesterol Lowering Efficacy of MK-0616, an Oral PCSK9 Inhibitor. LBS06, AHA Scientific Sessions 2021, 13–15 November.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« REVERSE-IT: Interim analysis shows promising effect of bentracimab on ticagrelor reversal Next Article
Remote healthcare programme improves hypertension and lipid control »
« REVERSE-IT: Interim analysis shows promising effect of bentracimab on ticagrelor reversal Next Article
Remote healthcare programme improves hypertension and lipid control »
Table of Contents: AHA 2021
Featured articles
The scope of remote healthcare in hypertension and hyperlipidaemia
Atrial Fibrillation
New developments in remote diagnostics and monitoring of AF
Head-to-head: Efficacy of dabigatran versus warfarin on cognitive impairment
Posterior left pericardiotomy safe and effective in reducing atrial fibrillation
LAA ligation did not reduce recurrent atrial arrhythmias in persistent AF
Equal benefits of early rhythm control in AF subtypes
CVD Risk Reduction
Remote healthcare programme improves hypertension and lipid control
Novel oral PCSK9 inhibitor shows promising results for hypercholesterolaemia
REVERSE-IT: Interim analysis shows promising effect of bentracimab on ticagrelor reversal
No significant effect of aspirin on reducing cognitive impairment
Milvexian phase 2 data supports safety and efficacy for VTE prevention after total knee replacement
Network meta-analysis observes no clear effect of eicosapentaenoic acid on CV outcomes
Heart Failure
Empagliflozin efficacious in HF patients with preserved ejection fractions ≥50%
EMPULSE: Empagliflozin improves outcomes of acute heart failure
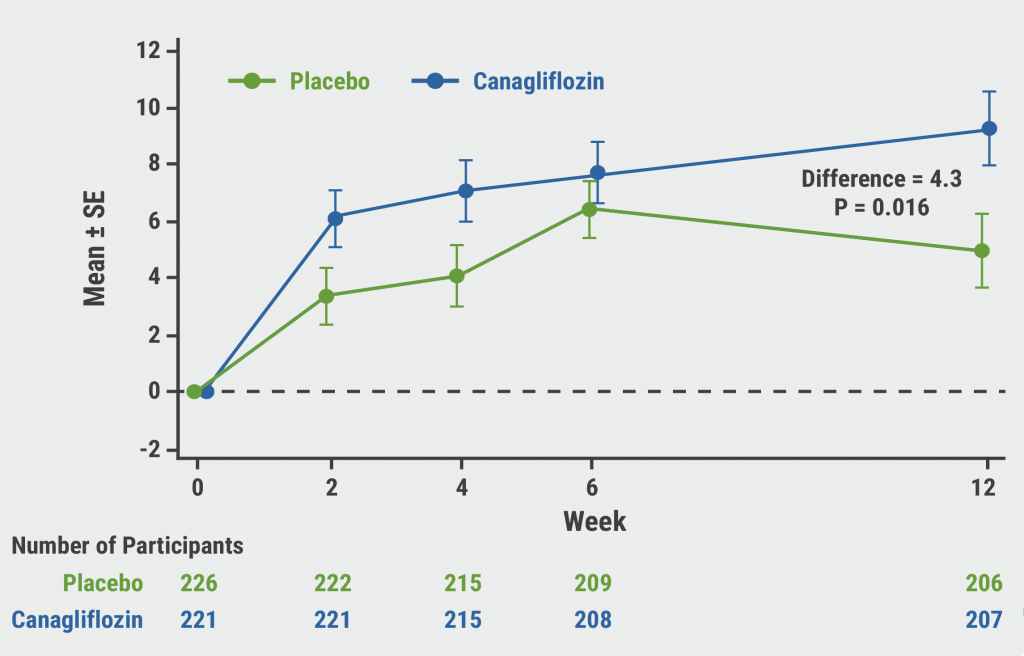
CHIEF-HF: Canagliflozin improves health status in heart failure
DREAM-HF: MPC therapy for HFrEF did not meet primary endpoint
Therapeutic approaches in heart failure with diabetes
Acute Coronary Syndrome
Ticagrelor cessation: early CABG non-inferior to delayed surgery
Distinguishing patients before AMI based on plaque morphology
Vascular Diseases: PVD
Rivaroxaban regimen beneficial after revascularisation for claudication
LIBERTY 360 shows quality-of-life improvements after peripheral vascular intervention
Deficient treatment outcomes after PVI in Black and low-income adults with PAD
REDUCE-IT: Cardiovascular risk reduction with icosapent ethyl in PAD
Vascular Diseases: CAD
Long-term reduced risk of CV events with ticagrelor plus aspirin after CABG
Early surgery outperforms conservative management in asymptomatic severe aortic stenosis
External support device for SVG grafts in CABG surgery shows promise
COVID-19 & the Heart
Blood pressure control disrupted during the pandemic
Icosapent ethyl did not reduce the risk of hospitalisation in COVID-19
Neutral effect of P2Y12 inhibitors in non-critical COVID-19 hospitalisations
COVID-19 mRNA vaccination benefits outweigh the risk for myocarditis
Other
2021 Guideline for Chest Pain: Top 10 takeaways
Accurate ejection fraction assessment in paediatric patients via artificial intelligence
Concomitant tricuspid annuloplasty reduces treatment failure in moderate tricuspid regurgitation
Related Articles
January 14, 2022
No significant effect of aspirin on reducing cognitive impairment
January 14, 2022
Therapeutic approaches in heart failure with diabetes
January 14, 2022
CHIEF-HF: Canagliflozin improves health status in heart failure
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

